Substances that chemically react with each other are called reagents and reactants. Reactants are involved in all chemical reactions.
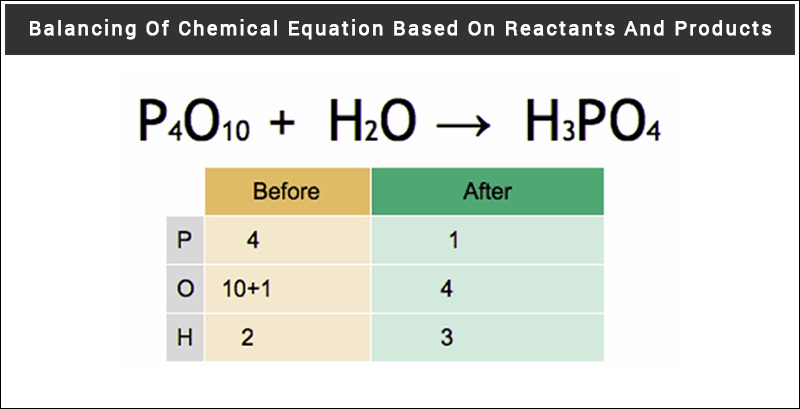 Chemical Equation Reactants And Products In Chemical Reactions
Chemical Equation Reactants And Products In Chemical Reactions
In a chemical reaction the atoms and molecules produced by the reaction are called products.
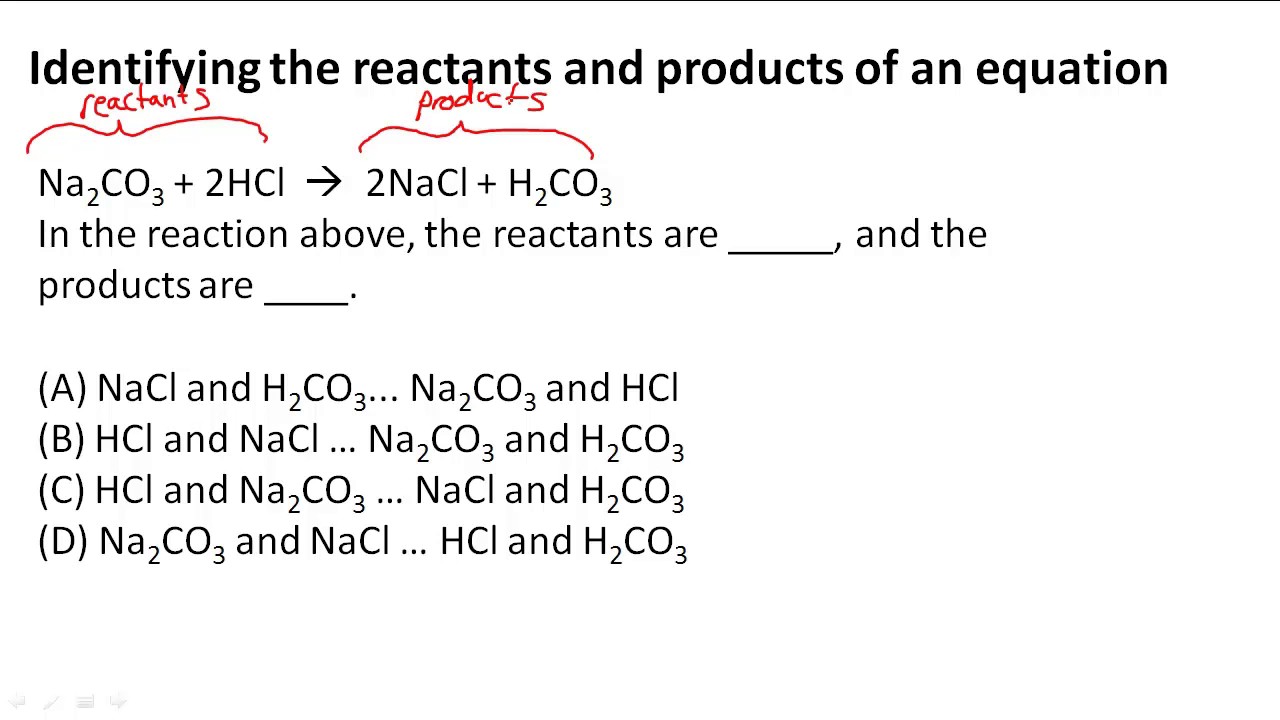
What are reactants in a chemical reaction. Necessity in Chemical Reactions. Catalysts work by increasing the frequency of collisions between reactants altering the orientation of reactants so that more collisions are effective reducing intramolecular bonding within reactant molecules or donating electron density to the reactants. Products are substances that are produced in the reaction.
In other words not all chemical reactions necessarily required a chemical reagent. The chemical bonds in reactant compounds are broken in order to form new bonds making a new compound. No new atoms are created and no atoms are destroyed.
The active metals calcium and sodium both react with water to form hydrogen gas and a base. Sodium and chloride ions for example are the reactants in the production of table salt. Chemical Reactions and Chemical Equations The atoms and molecules that interact are called the reactants.
It is a necessary component of a chemical reaction. The arrow is read as the word yields. The atoms in the reactants have been rearranged into new compounds the products.
Reactants are substances that start a chemical reaction. A chemical reaction is often accompanied by a temperature change bubbles color change andor precipitate formation. The products are carbon dioxide gas and water vapor.
The one or more substances produced by a chemical reaction are called the product. All chemical reactions begin with a reactant the general term for the one or more substances that enter into the reaction. This new compound is called the product of the reaction.
The tip of the arrow points in the direction in which the reaction proceeds. The properties of the products are different from those of the reactants. In a chemical formula the reactants are on the left side of the arrow and the products are on the right.
This means that the total mass of the reactants. In a chemical reaction only the atoms present in the reactants can end up in the products. The atoms and molecules produced by the reaction are called products.
When a candle burns the reactants are fuel the candlewick and wax and oxygen in the air. They consist of chemical or structural formulas of the reactants on the left and those of the products on the right. A reaction can occur even without a chemical reagent.
In a chemical reaction the reactants are the elements present when the reaction begins and the products are the elements and compounds produced as a result of the reaction. Instead you create a new substance with chemical reactions. Reactants are substances initially present in a chemical reaction that are consumed during the reaction to make products.
They are separated by an arrow which indicates the direction and type of the reaction. React together are called the reactants are formed in the reaction are called the products No atoms are created or destroyed in a chemical reaction. Reactants are consumed in the reaction.
You cant change one element into another in a chemical reaction that happens in nuclear reactions. In a chemical reaction the atoms and molecules that interact with each other are called reactants. For example burning methane in oxygen is irreversible.
Reactants are chemical species that act as the starting material of a chemical reaction. Vinegar and baking soda are reactants when you mix them together they bubble up and make really good lava for a model volcano. These reactions are said to be irreversible.
Other examples of everyday life chemical reactions include. During a chemical reaction the reactants are used to make the products. Chemical equations are used to graphically illustrate chemical reactions.
Rusting of iron burning of wood photosynthesis and combustion in the cars. A simulation about reactants products and leftovers httpphetcoloradoeduensimulationreactants-products-and-leftovers. Some chemical reactions go to completion resulting in all of the reactants becoming products.
A chemical reaction is a process in which one or more substances also called reactants are converted to one or more different substances known as products. Reactants are consumed in a chemical reaction. Reactants are consumed during the progression of a chemical reaction.
Reactants are the starting material that undergoes changes during a chemical reaction. A reactant is a substance that changes in a chemical reaction. In a chemical reaction substances elements andor compounds called reactants are changed into other substances compounds andor elements called products.
A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products. At the end of the reaction none of the reactants may be present in the reaction mixture but sometimes some of the reactants may be present at the end. The new substance that is formed is called the product of the reaction.
Catalysts eg enzymes lower the activation energy of a chemical reaction and increase the rate of a chemical reaction without being consumed in the process. The Chemical Nature of the Reacting Substances For example when small pieces of the metals iron and sodium are exposed to air the sodium reacts completely with air overnight whereas the iron is barely affected. Substances are either chemical elements or compounds.