The nonmetals in the periodic table. They are the second largest class of elements after metals.
 List Of Non Metals With Symbols And Their Uses In Periodic Table
List Of Non Metals With Symbols And Their Uses In Periodic Table
Hydrogen helium carbon nitrogen oxygen fluorine neon phosphorus sulfur chlorine argon selenium bromine krypton iodine xenon and radon.
What elements are nonmetals. Some nonmetals are liquids. When they form ions they gain electrons. It is yellow and not shiny at all.
Examples of nonmetals include. Hydrogen sometimes considered an alkali metal. Properties of nonmetals include a relatively low boiling point so many nonmetals are gases.
Hydrogen helium oxygen nitrogen fluorine neon or radon and many others. What is called ductility. Examples of nonmetals include hydrogen carbon chlorine and helium.
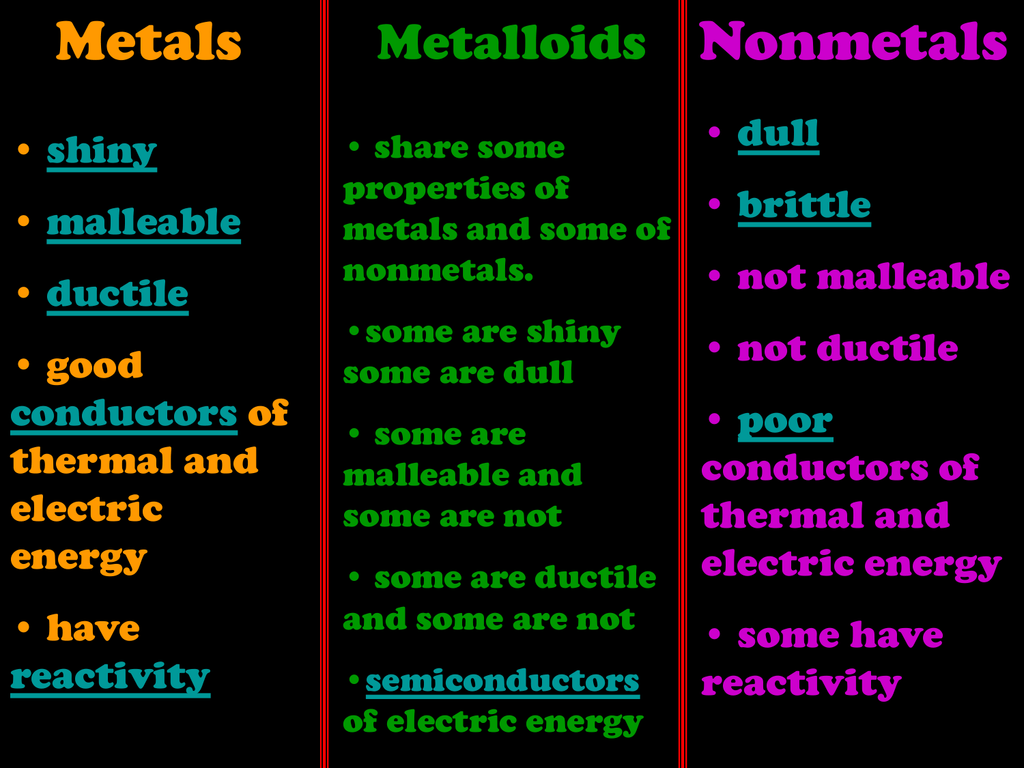
Not lustrous dull appearance. They are known to be lustrous or shiny highly malleable or ductile and are very good conductors of electricity and heat. In chemistry a nonmetal or non-metal is a chemical element that mostly lacks the characteristics of a metalPhysically a nonmetal tends to have a relatively low melting point boiling point and densityA nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivityChemically nonmetals tend to have relatively high ionization energy.
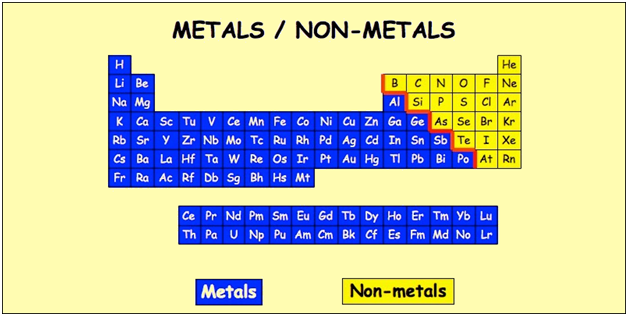
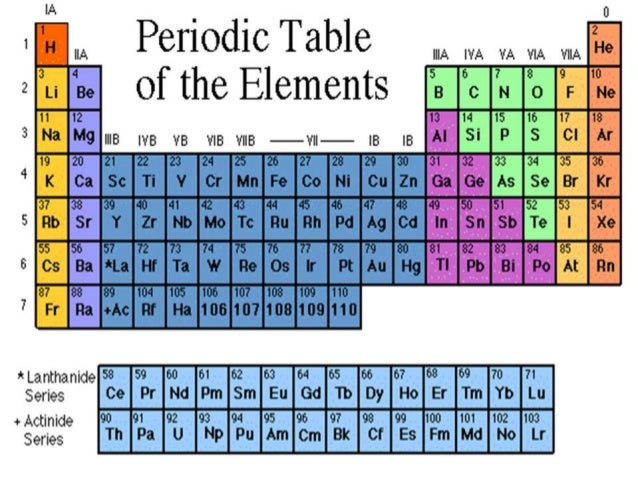
Nonmetals are separated from metals by a line that cuts diagonally through the region of the periodic table containing elements with partially filled p orbitals. Nonmetals are elements that generally cannot conduct electricity. Are good conductors of heat and electricity.
What elements are nonmetals. Some are gases including. The good news is that most elements are metals.
The chart below outlines three basic physical properties used to classify elements as metals nonmetal and metalloids. Click card to see definition. Nonmetals are fairly brittle and dull in appearance.
An example of a solid that is a nonmetal is sulfur. Elements on this line are metalloids or semimetals which have properties intermediate between those of metals and nonmetals. Property Luster Malleability Conductivity Other Properties Metals High metallic luster shiny Very malleable can be ham mered into thin sheets.
The halogens and noble gases are nonmetals but the nonmetal element group usually consists of the following elements. Nonmetals include the nonmetal group the halogens and the noble gases. When we study the elements it is important to know which elements are metals and which ones are not.
These elements have similar chemical properties that differ from the elements considered metals. Click again to see term. Every element to the right of this line is a nonmetal and all other elements most elements are metals.
Metals are substances that tend to donate electrons. The nonmetal element group is a subset of these elements. Nonmetals are found on the right side of the periodic table.
They also do not conduct electricity or. The elements that are generally considered other nonmetals include hydrogen carbon nitrogen phosphorus oxygen sulfur and selenium. And have at least one basic oxideMetalloids are metallic-looking brittle solids that are either.
D all of the above. These elements are shown in the following figure. Form alloys with other metals.
Chemically hydrogen carbon nitrogen oxygen phosphorus arsenic and selenium are the non-metallic elements in the periodic table. A simple video explains the meaning of elements and their classificationelements may be metals or non-metals and here you will identify the difference betw. Elements are classified as metals nonmetals or metalloids.
Nonmetal s or non-metal s are chemical elements that does not have the properties of a metal. Tap card to see definition. If you are trying to learn to distinguish between metals and non-metals a list and their uses is a good way to break them down and help memorize the difference between the two.
The 17 nonmetal elements are. Elements that are nonmetals are hydrogen carbon nitrogen phosphorus oxygen sulfur selenium all of the halogens and the noble gases. There are 7 elements that belong to the nonmetals group.
Oxygen sulfur and selenium are included in the subgroup chalcogens. The nonmetals are brittle not malleable or ductile poor conductors of both heat and electricity and tend to gain electrons in chemical reactions. The nonmetal element group consists of hydrogen carbon nitrogen oxygen phosphorus sulfur and selenium.
The chemical elements can be broadly divided into metals metalloids and nonmetals according to their shared physical and chemical propertiesAll metals have a shiny appearance at least when freshly polished. Non-metals are natural materials that do not produce heat or electricity and that are structurally brittle can not be easily rolling moulding extruding or pressing. Nitrogen and phosphorus are included in the subgroup pnictogens.
Examples of metals are Gold Silver Iron Uranium and Zinc. Nonmetals are elements showing less or no metallic properties.
Nonmetals display some or all of the following characteristics.
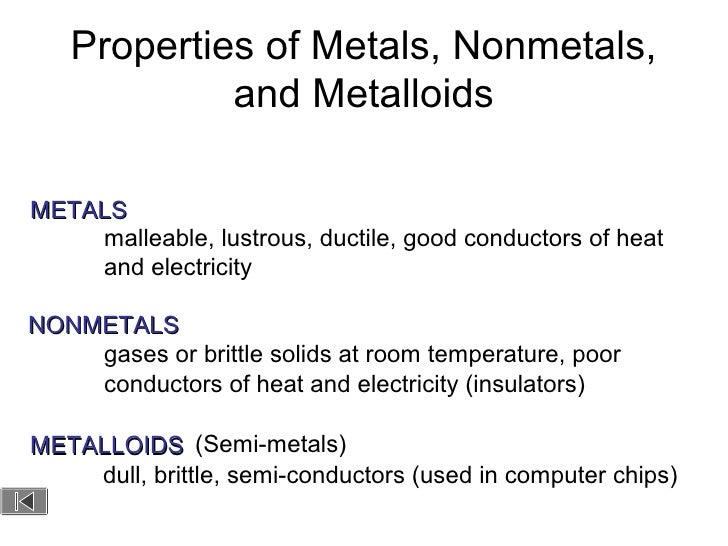
Properties of metals nonmetals and metalloids. All metals have a shiny appearance at least when freshly polished. Nonmetal are poor conductors of electricity and heat. Metalloids have properties intermediate between the metals and nonmetals.
Trends based on Groups. Development of the Periodic table Effective Nuclear Charge Atomic and Ionic sizes Ionization Energy Electron Affinity Metals Nonmetals and Metalloids. Metalloids share characteristics of both metals and non-metals and are also called semimetals.
They are less conductive than metal. Metals and metalloids are generally solid at room temperature whereas nonmetals exist as liquids gases or brittle solids. Nonmetals does not reflect light.
Metalloids have properties that are between the properties of nonmetals and metals. Non-metals do not conduct heat or electricity very well. List four physical properties of metals nonmetals and metalloids.
Boron silicon germanium arsenic antimony and tellurium are the most commonly recognized metalloids. List three properties of this substance. Metalloids exhibit some properties of metals as well as of non-metals.
Mostly solids at room temperature. They are also called semimetals. Metalloids are elements found between the metals and nonmetals on the periodic table of the elements.
These elements are shown in the following figure. Whether bonding with other metalloids or joining with metals or nonmetals the dance of a metalloid is fully determined by the rules set by electronegativity. Good conductors of heat and electricity.
The main difference between metals non-metals and metalloids are that metals are elements that are hard malleable fusible shiny ductile and good conductors. Metalloids are brittle solids that crumble to powder when struck. An appearance that is similar to metals.
Form alloys with other metals. Very brittle break easily. The nonmetals are brittle not malleable or ductile poor conductors of both heat and electricity and tend to gain electrons in chemical reactions.
Are good conductors of heat and electricity. Properties of Nonmetals. Properties of metals nonmetals metalloids.
Poor conductors of heat and electricity. Some nonmetals are liquids. Examples of nonmetals include oxygen chlorine and argon.
Metals have a shiny appearance non-metals have a dull appearance. Nonmetals pull the metals to them guiding them strictly like a conductor that leads a orchestras rehearsal. Metalloids are all solid at room temperature.
The difference in physical properties of Metals Nonmetals and Metalloids The difference in Properties of Metals Nonmetals and Metalloids State. Chemical Properties of Metalloids Their physical properties tend to be metallic but their chemical properties tend to be non-metallic. Nonmetals have very distinct properties than those of metals and metalloids.
The metalloids elements like boron silicon and antimony have some properties of metals and other properties of nonmetals. Usually less dense compared to metals. Malleability Metals can withstand hammering and can be shaped into thin sheets such as foils.
Terms in this set 23 malleable. They are more brittle than metals. These elements run diagonally across the Periodic Table.
The nonmetals in the periodic table. Physical Properties of metalloids Can be shiny or dull Conductivity of heat and electricity better than nonmetals but not as good as metals. Complete opposite of a metal.
Solid at room temperature. Trends in Metallic and Nonmetallic Character. Typically nonmetals have a dull appearance since they do not have a metallic appearance.
They can form alloys with other metals. Example non-metal elements are Hydrogen and Carbon. Metalloids are uniquely flexible partners.
Metalloids are useful in the semiconductor industry. When you think of nonmetals you might think of materials made of plastic or polystyrene. Non-metals do not have properties present in metals whereas metalloids are elements that have intermediate properties of both metals and non-metals.
Non-metals are typically brittle and are not easily molded into shapes. Metalloids are metallic-looking brittle solids that are either semiconductors or exist in semiconducting forms and have amphoteric or weakly acidic oxides. The oxidation number of an element in this group can range from 3 to -2 depending on the group in which it is located.
They are not lustrous. Nonmetals exhibit very different properties from metals. Have luster shiny metal.
Unlike metals nonmetals are not malleable or ductile. Metalloids are not malleable. Metals Nonmetals and Metalloids Chemistry 101 Periodic Table properties.
Nonmetals have properties opposite those of the metals. Metals are located in s p d and f blocks in the periodic table though non-metals is located in s and p blocks and metalloids are located in p block of the periodic table. The chemical elements can be broadly divided into metals metalloids and nonmetals according to their shared physical and chemical properties.
However metalloids have a shiny and dull appearance. Left of the stair step line. Hence they are also known as semi-metals.
Worksheets are metals and non metals chapter 3 metals nonmetals and metalloids teks lesson metals nonmetals and metalloids classifying metals nonmetals and metalloids nonfiction reading test metal detectors compounds formed between metal non metals metal natural science and technology grade 5. Compared to metals nonmetals are less dense. And have at least one basic oxide.
Property Metals Nonmetals Metalloids Luster Metals reflects light from their surface and can be polished for example in gold silver and copper.
For example sulfur and carbon are both non-metals. An Introduction to General Organic and Biological Chemistry 12th Identify each of the following elements as a metal a nonmetal or a metalloid.
 Metals Nonmetals And Metalloids
Metals Nonmetals And Metalloids
Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals.
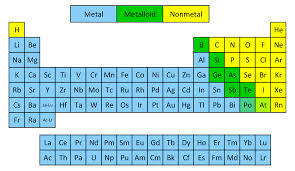
Metals and nonmetals metalloids. Take up the quiz below and find out. Typical nonmetals have a dull coloured or colourless appearance. Are poor conductors of heat and electricity.
Introduction to Metals Non Metals and Metalloids. Trends based on Groups Formal Charges 99 Practice Problems. SOLIDS LIQUIDS or GASSES.
Somewhere between metals and nonmetals lie metalloids or semiconductors Let us identify the. What factors should we consider while distinguishing these three. Metalloids tend to be economically important because of their unique conductivity properties they only partially conduct electricity which make them valuable in the semiconductor and computer chip industry.
Metalloids Some elements between the metals and non-metals in the periodic table have properties which are a mixture of the properties of metals and non-metals. VINotebook Metals Non-metals MetalloidsMpptx - Metals Nonmetals and Presented by Kesler Science Metalloids Vers 072020 u00a9 Kesler Science LLC Reflect. Metals nonmetals and metalloids are elements that are found in the earth.
These elements are called metalloids. Most or some elements in each category share a range of other properties. A few elements have properties that are either anomalous given their category or otherwise extraordinary.
What is the difference between them. Metalloids are metallic-looking brittle solids that are either semiconductors or exist in semiconducting forms and have amphoteric or weakly acidic oxides. And have acidic oxides.
Where is between metals and metalliods. 1 This group of elements is made up of all different states of matter solid liquid and gas a Nonmetals b Metals c Metalloids 2 These elements always have a shiny luster a Nonmetals b Metals c Metalloids 3 These elements are located in a stair step pattern on the periodic table a Nonmetals b Metals c Metalloids 4 Elements in this group are good conductors of heat AND. In chemistry we learn about metals non-metals and metalloids.
Are brittle when solid. They react with oxygen to form sulfur dioxide and carbon dioxide. The metalloids or semimetals have properties that are somewhat of a cross between metals and nonmetals.
The main difference between metals nonmetals and metalloids is that metals show the highest degree of metallic behavior and nonmetals do not show metallic behavior whereas metalloids show some degree of metallic behavior. The main difference between metals non-metals and metalloids are that metals are elements that are hard malleable fusible shiny ductile and good conductors. Discussion of the properties of each.
Non-metals do not have properties present in metals whereas metalloids are elements that have intermediate properties of both metals and non-metals. The exception is hydrogen H the first element on the periodic table. Metals can be found on the left side of the periodic table while nonmetals are found on the right side.
Metals Nonmetals and Metalloids. Where at the metalloids located on the Periodic Table. Metals form oxides that are basic but non-metals form oxides that are acidic.
Most of these elements are used in various applications. Elements to the left of the line are considered metals. Can a metalloid have properties of metals and nonmetals.
Mercury a metal has a low melting point and exists as a liquid at room temperature graphite a form of carbon a non-metal has a high boiling point and is also a good conductor of electricity A. Elements to the far right of the periodic table are nonmetals. Questions and Answers 1.
Metals are _____ and can be drawn into a wire.
Nonmetals with the exception of hydrogen are located on the right side of the periodic table. The noble gases are almost completely inert.
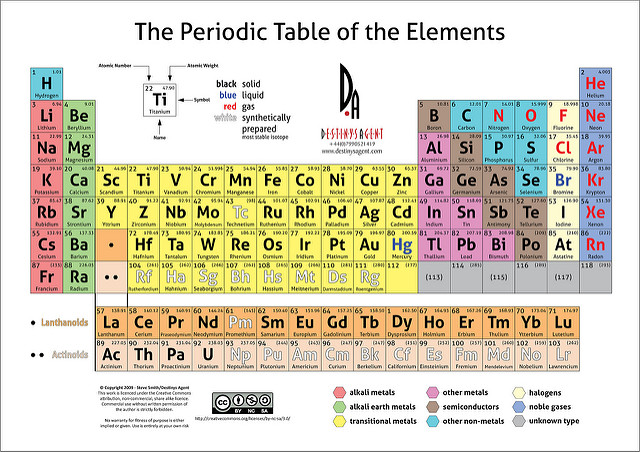 How Can You Differentiate Between Metals And Nonmetals On The Periodic Table Socratic
How Can You Differentiate Between Metals And Nonmetals On The Periodic Table Socratic
Nonmetals are located on the right side of the periodic table.

Where are the nonmetals on the periodic table. This line is often referred to as the staircase because of its shape. The halogens and noble gases are nonmetals but the nonmetal element group usually consists of the following elements. These elements are shown in the following figure.
They are located to the right of the metalloids and to the left of the halogens. Polyatomic nonmetals have structures with either three nearest neighbours as is the case for example with carbon in its standard state of graphite or two nearest neighbours for example in the case of sulfur. Nonmetals have properties opposite those of the metals.
The nonmetal elements occupy the upper right-hand corner of the periodic table. The nonmetals are brittle not malleable or ductile poor conductors of both heat and electricity and tend to gain electrons in chemical reactions. Non-metals can be easily located on the Periodic Table because they are to the right of the line that looks like a stepping ladder.
Atoms to the left of the. Now you can also colour hydrogen H red. Colour all the blocks to the right of the semi-metals red.
Non metals in periodic table. These elements are often referred to as other nonmetals as the halogens and noble gases are also nonmetals. Non-metals are characterized by having the exact opposite properties of metals.
Hydrogen is a nonmetal which is located on the left top corner of the Periodic table. The only nonmetal that is a liquid at room temperature is _____. The non metalsin periodic table are mainly in the upper right position The only chemical element that is not found in this region of the table is Hydrogen which is located in the upper left corner together with Alkaline Metals but since it behaves in most circumstances as a Non-Metal it is classified as such.
All these elements are non-metals. ADVERTISEMENT When we study the elements it is important to know which elements are metals and which ones are not. Moving rightward across the standard form of the periodic table nonmetals adopt structures that have progressively fewer nearest neighbours.
The nonmetals are located on the upper right side of the periodic table. Metals are lustrous good conductors of electricity and readily shaped they are ductile and malleable whereas solid nonmetals are generally brittle and poor electrical conductors. From left to right in the periodic table the nonmetals can be divided into the reactive nonmetals and the noble gases.
The nonmetals are in the last few columns of the periodic table because they have from 4-8 electrons is the outermost shell putting them groups 13-18 on the periodic table. Metals are located on the left of the periodic table and nonmetals are located on the upper right. Nonmetals are separated from metals by a line that cuts diagonally through the region of the periodic table containing elements with partially filled p orbitals.
Non-Metals In The Periodic Table. The nonmetals are a group of elements in the periodic table. The only exception to this is atomic number 1 Hydrogen H which has a different location on the table.
Nonmetals include the nonmetal group the halogens and the noble gases. Most of the elements that make up the human body are nonmetals. The nonmetals in the periodic table.
Elements that are nonmetals are hydrogen carbon nitrogen phosphorus oxygen sulfur selenium all of the halogens and the noble gases. Nonmetals are located on the upper right side of the Periodic table see above image. These elements have similar chemical properties that differ from the elements considered metals.
Some nonmetals are liquids. The reactive nonmetals near the metalloids show some incipient metallic character such as the metallic appearance of graphite black phosphorus selenium and iodine. Atomic structure and the periodic table Elements in group 1.
Most periodic tables print a thick black line to show the division between metals and nonmetals. Nonmetals on the Periodic Table. They are separated by a diagonal band of semimetals.
Nonmetals are located on the far right side of the periodic table except hydrogen which is located in the top left corner. Metals Nonmetals and Metalloids on the Periodic Table - YouTube A description and practice of finding metals nonmetals and metalloids on the Periodic TableIn general metals are found on the. Non-metal elements are on the right of the stepped line Metals are on the left of the periodic table and non-metals are on the right.
Well you have got the answer of Where are Nonmetals located on the Periodic Table. The nonmetal element group is a subset of these elements. On most versions of the Periodic Table hydrogen is placed with the metals even though it has physical properties similar to those of the non-metals it is a gas at room temperature.
The nonmetals list which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc. Nonmetals react by gaining electrons rather than losing electrons because they have a lot of electrons on the outermost shell making is easier to gain a few electrons.
Metals are strong a good conductor of heat and electricity ductile since they can be drawn into wire and malleable since it can be hammered or rolled into sheets. The position of an element provides information about its properties.
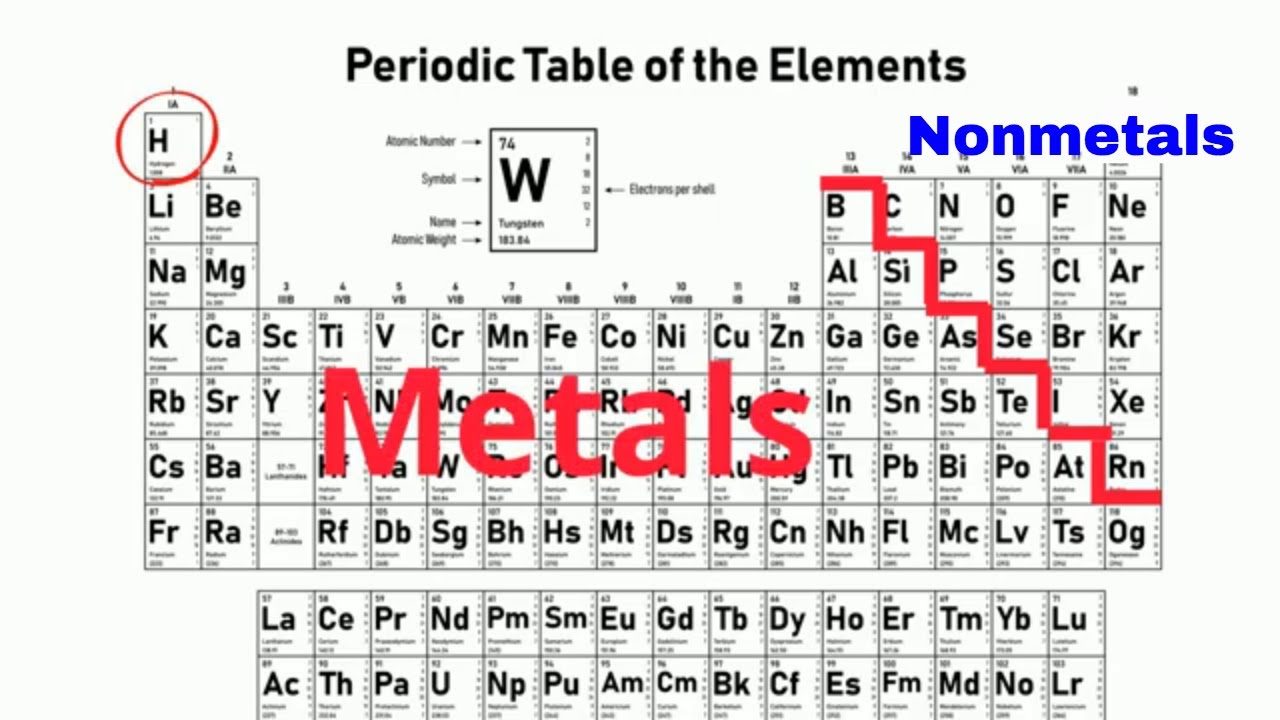 How To Identify Metals Nonmetals And Metalloids On The Periodic Table Youtube
How To Identify Metals Nonmetals And Metalloids On The Periodic Table Youtube
Learn about the metals nonmetals and metalloids and the periodic table.

Metals and nonmetals periodic table. Some allotropes particularly those of elements located in periodic table terms alongside or near the notional dividing line between metals and nonmetals exhibit more pronounced metallic metalloidal or nonmetallic behaviour than others. The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. These elements have similar chemical properties that differ from the elements considered metals.
The metals are found on the left and the nonmetals are found on the right. Nonmetals include the nonmetal group the halogens and the noble gases. The periodic table The elements can be placed in the periodic table.
The existence of such allotropes can complicate the classification of the elements involved. Elements just to the left of the line may be termed metalloids or semimetals and have properties intermediate between those of the metals and nonmetals. This includes the alkali metals alkaline earth metals transition metals lanthanides and actinides.
The nonmetals or non-metals are a group of elements located on the right side of the periodic table except for hydrogen which is on the top left. Using it you should be able to classify all the elements in different ways. Nonmetals have properties opposite those of the metals.
These elements are shown in the following figure. Most elements are metals with different properties to. The nonmetal element group consists of hydrogen carbon nitrogen oxygen phosphorus sulfur and selenium.
KS3 new for the Activate 2 resource The Periodic Table unit. Differentiate metals from non-metals. The Periodic Table contains a lot of useful information on the elements.
Metals are grouped on the left side of the periodic table with an exception of a hydrogen atom. Metals nonmetals and metalloids make up the periodic table with metals constituting the large majority of all metals. The nonmetals in the periodic table.
The reactive nonmetals near the metalloids show some incipient metallic character such as the metallic appearance of graphite black phosphorus selenium and iodine. Please give constructive feedback D. Metals are on the left of the periodic table and non-metals are on the right.
The nonmetals lis t which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc. Metals and nonmetals are differentiated on a periodic table because they have different reactivity. The nonmetals are brittle not malleable or ductile poor conductors of both heat and electricity and tend to gain electrons in chemical reactions.
Non-metals are grouped on the right side of the periodic table. These elements and those to the right of them are nonmetals. Metals and non-metals are elements in the periodic table.
The periodic table of metals and nonmetals can be broken down to give you a sense of each elements characteristics. Some nonmetals are liquids. Atoms of group 1 elements have one.
And metalloids are the borderline. Metals tend to lose electrons to nonmetals or form metallic bonds with other metals. Metals oxides rust non-metals periodic table reactions.
Similar structure to my other power points following the input - activity - review phasing plenary sections for progress checking Clear learning objectives and outcomes Modern and engaging layout Little adaptation needed. The nonmetal elements occupy the upper right-hand corner of the periodic table. They differ from the conduction of heat and electricity appearance state at temperature and strength.
Atomic structure and the periodic table Elements in group 1 and group 2 are metals. The nonmetal element group is a subset of these elements. Some nonmetals C black P S and Se are brittle solids at room temperature although each of these also have malleable pliable or ductile allotropes.
On the periodic table metals are separated from nonmetals by a zig-zag line stepping through carbon phosphorus selenium iodine and radon. From left to right in the periodic table the nonmetals can be divided into the reactive nonmetals and the noble gases. Full lesson ready to use straight out of the box.