Diffusion is the movement of a substance from an area of higher concentration to an area of lower concentration. According to this Law the rate of Diffusion of different gases at a constant temperature is inversely proportional to the square root of its density.
 Understanding Graham S Law Of Effusion Youtube
Understanding Graham S Law Of Effusion Youtube
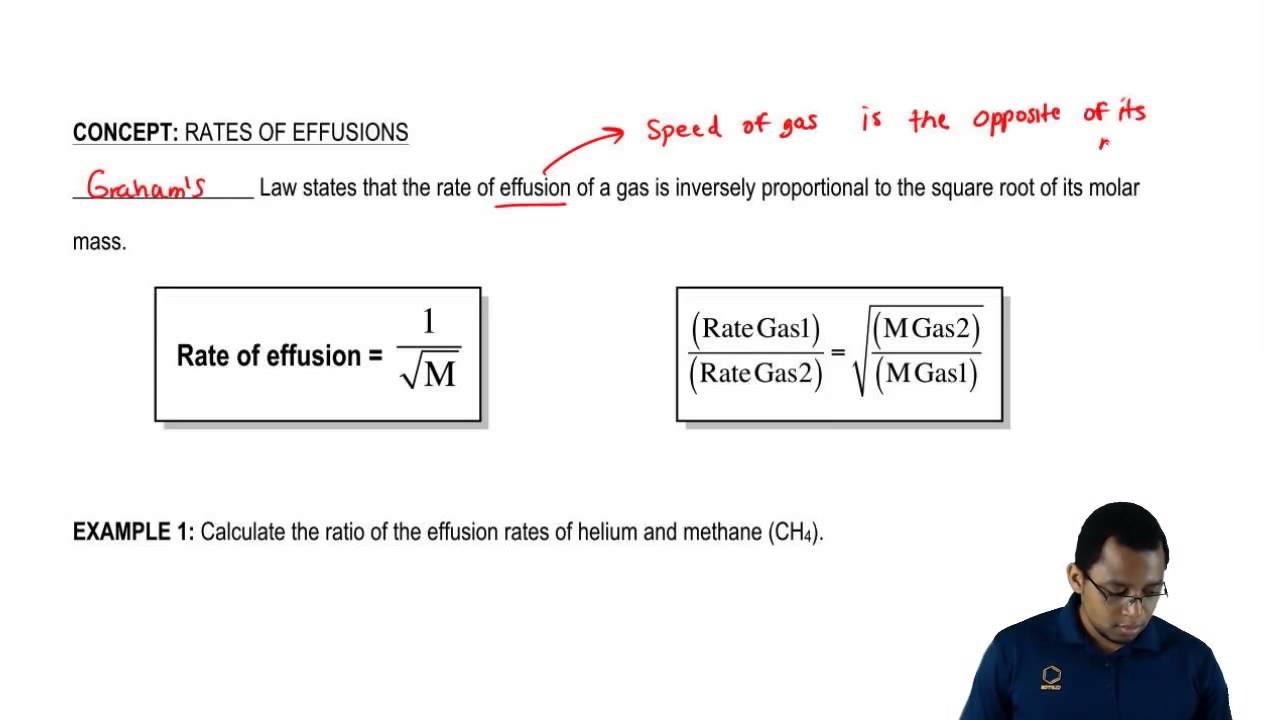
Graham found experimentally that the rate of effusion of a gas is inversely proportional to the square root of the mass of its particles.

Graham's law of effusion. In1829 Thomas Graham a Scottish Chemist formulated the Grahams Law of the Diffusion and Effusion of Gases. In Grahams Law we will look at the rate of effusion movement of gas through a small pinhole into a vacuum more often than we will look at a speed like a root mean square speed. Rate 1 Rate 2 M 2 M 1.
Grahams law states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molar mass. But if time is used the equation changes Grahams Law deals with the effusion of gases. Thomas Graham experimented with the effusion process and discovered an important feature.
But one of the most important is the frequency with which molecules collide with the interior surface of the balloon. Grahams Law of Effusion Grahams law states that the rates of effusion of two gases are inversely proportional to the square roots of their molar masses at the same temperature and pressure. A common example of effusion is the loss of gas inside of a balloon over time.
It helps in the separation of isotopes of certain elements. He also gave the rate at which molecules would escape ie. He established the relationship through experiments.
Grahams law also applies to effusion the process in which gas molecules flow through a small hole in a container. Gas molecules that are lighter will travel faster than the heavier gas molecules. Grahams law states that the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molecular weight.
Graham Law The rate of effusion of a gaseous substance is inversely proportional to the square root of its molar mass. Grahams law of effusion also called Grahams law of diffusion was formulated by Scottish physical chemist Thomas Graham in 1848. Effusion is the process of spreading of gas in open volume through tiny hole Grahams law state that at constant temperature and pressure rate of diffuse is inversely proportional to the square root of their molecular mass or vapor density During extraction of uranium from earth crust it is present in two isotopic forms U 238 and U 235.
This means light gasses effusediffuse quickly and heavier gases effusediffuse slowly. See this law in equation form below. Effusion is the process that occurs when a gas is permitted to escape its container through a small opening.
This law was developed by the physical chemist Thomas Graham in 1848. Grahams law states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molar mass. R M½ constant.
Grahams Law which is popularly known as Grahams Law of Effusion was formulated Thomas Graham in the year 1848. The rate at which gases will effuse from a balloon is affected by a number of factors. In these equations r rate of diffusion or effusion and M molar mass.
This formula can be written as. The rate of diffusion. Grahams law states that the rate at which a gas will effuse or diffuse is inversely proportional to the square root of the molar masses of the gas.
Grahams Law of Effusions says that the lighter a gas particle is the faster it will moveIn this video we will learn the difference between effusion and dif. Importance of Grahams law of diffusion - definition It helps in the separation of gases having different densities. Formula for Grahams Law of Diffusion and Effusion r 1 M½.
According to Grahams Law at constant pressure and temperature molecules or atoms with lower molecular mass will effuse faster than the higher molecular mass molecules or atoms. Diffusion occurs spontaneously on its own. A fundamental law of radiation biology that states that the radiosensitivity of a tissue is increased the greater the number of undifferentiated cells in the tissue the greater the mitotic activity and the greater the length of time that they are actively proliferating.
This empirical law was stated by Scottish chemist Thomas Graham in 1848. Grahams law of Effusion or diffusion states that when the temperature and pressure are constant than atoms with high molar mass effuse slower than atoms with low molar mass. Grahams law of diffusion or Grahams law of effusion is a law that expresses the relationship between the rate of diffusion or effusion to molar masses of particles.
Effusion is defined as a loss of material across a boundary. Grahams law is an empirical relationship that states that the ratio of the rates of diffusion or effusion of two gases is the square root of the inverse ratio of their molar masses. That means we are mostly looking at amounts that move per unit time not how fast the individual particles are moving.
R 1 M½.