For example powdered iron and powdered sulfur mixed together makes a mixture of iron and sulfur. Pure substances cannot be separated into any other kinds of matter while a mixture is a combination of two or more pure substances.
Distinguish Between Pure Substance And Mixture Cbse Class11 Chemistry 2016
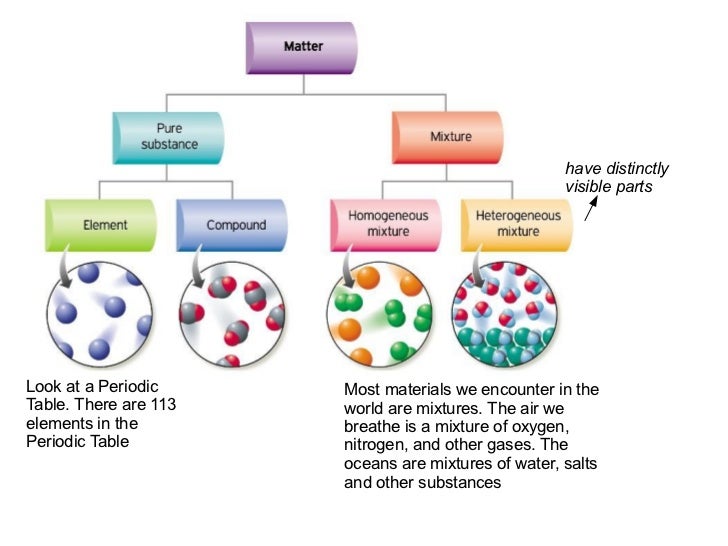
Matter can be broken down into two categories.
How can you distinguish a substance from a mixture. 1 Pure substance The substances that contain only one type of particle and they are free from any mixture are known as pure substances. A mixture is composed of different types of atoms or molecules that are not chemically bonded. The chemical properties of the components dont change.
While the compound is a pure substance the mixture is an impure substance. A mixture is made from different substances that are not chemically joined. Since they are formed by ionic procedures they can only be divided by ionic operations equally.
Therefore the properties are uniform throughout the sample. A paper chromatogram can be used to distinguish between pure and impure substances. Mixtures are physically combined structures that can be separated into their original components.
Separation by chromatography produces a chromatogram. A homogeneous mixture sometimes called a solution is relatively uniform in composition. However matter can also be classified into pure substances and mixtures.
In a sample of impure salol salol mixed with other substances the temperature falls gradually as it freezes. A chemical substance is composed of one type of atom or molecule. A pure substance has constant physical and chemical properties while mixtures have varying physical and chemical properties ie boiling point and melting point.
Mixtures are physically combined structures that can be separated into their original components. What you need to know. 2021s latest celeb couple.
Substances are often regarded as being uncontaminated to differentiate them from mixtures and they cannot be split into more than one tangible entity. DiCaprios beach house was full of Titanic memorabilia. The difference between a pure substance and a mixture is that a pure substance such as aluminum has one type of particle in it and mixture such as salt has two or more particles within it or.
Difference Between Pure Substance And Mixture Solid liquid and gas are the three states of matter. Its basically a particular kind of matter with uniform properties. Mixtures melt over a range of temperatures The melting point range of a substance is the temperature range from which the first crystal starts to melt to the temperature at which the last crystal.
Pure substances and mixtures. Gold silver iron and aluminium are pure substances to name a few. It is difficult for many science students to understand the difference between compound and mixture so here weve simplified it for you.
Mixtures can be either homogeneous or heterogeneous. Substances which have a specific composition and cannot be separated into any constituents are called pure substances. Mixtures can be separated by physical methods.
You can do this by adding water dissolving the salt and then filtering the mixture. Water is a substance and seawater is a mixturehow can you distinguish a mixture from a substance based on their boiling point. Pure substances are homogenous.
Messed up wiring needed for NFL. Generally substances can be subdivided into two groups. In contrast mixtures contain two or more substances so they can be separated.
Compared to pure substances mixtures composition can be changed by mixing different proportions of substances within it. Pure substances are further broken down into elements and compounds. The mixture is the physical combination of substances bonded together in any proportion.
If no chemical reaction occurs when two materials are mixed they form a mixture. A pure substance produces one spot on the chromatogram an. Mixtures are materials that contain two or more chemical substances dispersed among each other mixed together.
By their chemical composition pure substances get divided into two types elements and compounds. Mixtures can be heterogeneous and having a non-uniformity throughout the sample. You then end up with pure sand.
Mixtures show the properties of the pure substances in it. In a sample of pure salol the temperature stays the same as it changes state. While a pure substance cannot be separated physically the components of a homogeneous mixture can be separated through mechanical and thermal processes such as distillation sifting filtering and crystallization.
Every portion of the mixture is like every other portion. Substances if chemical is composed of one type of atom or molecule. Iowa lawmaker protests lack of mask mandate with jeans.
They can be separated.
Click to see full answer. A chemical substance can be solid liquid gas or plasma.
It doesnt have to consist of a single element or type of moleculePure hydrogen is a pure substance.
/examples-of-pure-substances-608350-v3-5b4cfc5646e0fb005b4d9588.png)
Is sugar a pure substance. Table sugar or sucrose is not a mixture. Sugar or sucrose is a compound with the chemical formula C 12 H 22 O 11. Two pure substances mixed together are known as a mixture.
Examples of pure substances that are crystals include salt diamond protein crystals and copper sulfate crystals. A substance can be anything. Let me start with a pure substance and work up from there.
Compounds such as water salt or crystals baking soda amongst others are also grouped as pure substances. All chemical compounds have a definite chemical composition and are thus classified as pure substances. If not you have a mixture.
Pure substances can be either elements or compounds. Pure sugar is an example of a pure substance. Scientists often use filtration to separate pure substances from a mixture in order to analyze the materials.
A substance can be anything. It is a mixture that combines mostly water sugar fat and proteins. Other chemical substances commonly encountered in pure form are diamond carbon gold table salt sodium chloride and refined sugar sucrose.
Sugar is a pure substance because it has a definite composition. Is sugar dissolved in water a pure substance. Even naturally occurring substances like crude oil are mixtures of many different substances.
Pure substances are made of only one type of atom or molecule. Let me start with a pure substance and work up from there. Table sugar or sucrose is not a mixture.
All elements are mostly pure substances. Glucose and there are no contaminants in the water or sugar before combining the two. A pure substance refers to an element or a compound that has no component of another compound or element.
Pure sugar is obtained from its solution in water by the process of crystallisation. If so you have a pure substance. Yes sugar is a pure substance because pure substances are In this case it is a compound.
Pure substances are often called pure to set them apart from mixtures. Sugar and water assuming sugar is all one type eg. All elements are pure substances.
A pure substance usually participates in a chemical reaction to form predictable products. Depending on who you talk to homogeneous mixtures may be considered examples of pure substances. Is sugar a pure substance or a compound.
Sugar is a pure substance because its composition is homogeneous throughout. Sugar salt and baking soda are pure substances that are compounds. Some examples are carbon iron water sugar salt nitrogen gas and oxygen gas.
In this case the substance contains two different molecules. Examples of Pure Substances. Is sugar a mixture or a pure substance.
It is a pure substance namely sucrose C12H22O11. So is pure honey even though it consists of many different types of molecules. However in practice no substance is entirely pure and chemical purity is specified according to the intended use of the chemical.
Most substances found in the world are not pure substances but are a combination of various chemicals and compounds. Since white sugar is sucrose C12H22O11 it is a pure substance. Compounds consist of two or more elements that have a specific ratio.
Elements are pure substances because they cannot be broken down any more. It is a compound. Sugar is a pure substance even though most people.
Elements are pure substances because they cannot be broken down any more. Pure substances cannot be separated into other substances. Its molecular formula is C12H22O11.
A few of them include gold copper oxygen chlorine diamond etc. It is a compound. Examples of pure substances include tin sulfur diamond water pure sugar sucrose table salt sodium chloride and baking soda sodium bicarbonate.
Depending on who you talk to homogeneous mixtures may be considered examples of pure substances. Hydrogen gas and pure iron are examples of pure substances. A pure substance is when only one type of atom or molecule is present.
In the real world it is probably impossible to get it 100 pure but table sugar is essentially a pure compound.
Soil is composed of small pieces of a variety of materials so it is a. Learn vocabulary terms and more with flashcards games and other study tools.
 Ppt Pure Substances Vs Mixtures Powerpoint Presentation Free Download Id 89827
Ppt Pure Substances Vs Mixtures Powerpoint Presentation Free Download Id 89827
Pure substances can be either elements or compounds.

Is sugar a mixture or pure substance. A pure substance can be either an element or a compound but the composition of a pure substance doesnt vary. Let me start with a pure substance and work up from there. Let me start with a pure substance and work up from there.
Examples of pure substances include tin sulfur diamond water pure sugar sucrose table salt sodium chloride and baking soda sodium bicarbonate. Which Plants Produce Sugar. Elements are pure substances because they cannot be broken down any more.
When a plant takes in carbon dioxide from the air and energy from the sun it forms sugar in its purest form. Sugar or sucrose is a compound with the chemical formula C 12 H 22 O 11. Sugar is a pure substance since it is the compound called glucose.
In the multiple-choice the substances that are not pure substances are pasteurized milk and clean airMilk is a mixture of water fats etc. If youre cooking and mix flour sugar and salt together to prepare for baking youve created a mixture because the ingredients have remained. Compounds consist of two or more elements that have a specific ratio.
In a fruit smoothie each individual ingredient like apples bananas strawberries sugar etc. Pop is a mixture. Some of the examples of pure substances can be pure water gold platinum etc whereas some of the examples of the mixtures can be carbon dioxide gas sugar alcohol etc.
Sugar is a pure substance because its composition is homogeneous throughout. When theyre joined the elements atoms are no longer made up of the same properties. Tin sulfur and diamond are examples of pure substances that are chemical elements.
It is a compound. Elements are pure substances because they cannot be broken down any more. Pure substances are often called pure to set them apart from mixtures.
What is the difference between a pure substance and a mixture. However in practice no substance is entirely pure and chemical purity is specified according to the intended use of the chemical. Pure alcohol could be ethanol methanol or a mixture of different alcohols but as soon as you add water which is not alcohol you no longer have a pure substance.
In the list the substances pure gold element. Is soil a substance or mixture. Like of it has a blender mixing together elements and compounds to get a mixture.
Sucrose or table sugar is a naturally-occuring substances that you can find in plants after theyve gone through photosynthesis. Other chemical substances commonly encountered in pure form are diamond carbon gold table salt sodium chloride and refined sugar. A mixture is a mix of elementscompounds.
Table sugar or sucrose is not a mixture. Mixtures are the substances that are made up of the combination of two or more substances and these substances can be separated by the physical and the chemical processes. More specifically each molecule of sugar has 12 carbon atoms 22 hydrogen atoms and 11 oxygen atoms.
Depending on who you talk to homogeneous mixtures may be considered examples of pure substances. It is a compound. The difference between a mixture and a pure substance is that a mixture is made up of 2 or more particles and a pure substance is made up of only one particle and one particle alone.
Contains two or more pure substance Mixtures can be any combination of solids liquids and gases Breads are mixtures of yeast flour sugar water air and other chemicals. Table sugar or sucrose is not a mixture. Start studying Pure Substances or Mixture.
The sugar is then extracted and prepared for use. A pure substance has a definite and constant composition like salt or sugar. Act as its own element or compound aka a pure substance.
If you dissolve sugar in water you will have a homogeneous mixture which is a solution. Pure sugar is a compound which is a pure substance. Crystals in general are pure substances.
Pure sugar is a compound which is a pure substance. Examples of pure substances include tin sulfur diamond water pure sugar sucrose table salt sodium chloride and baking soda sodium bicarbonate.
Pure substances A pure substance has a definite and constant composition like salt or sugar. Is _____ a pure substance or a mixture.
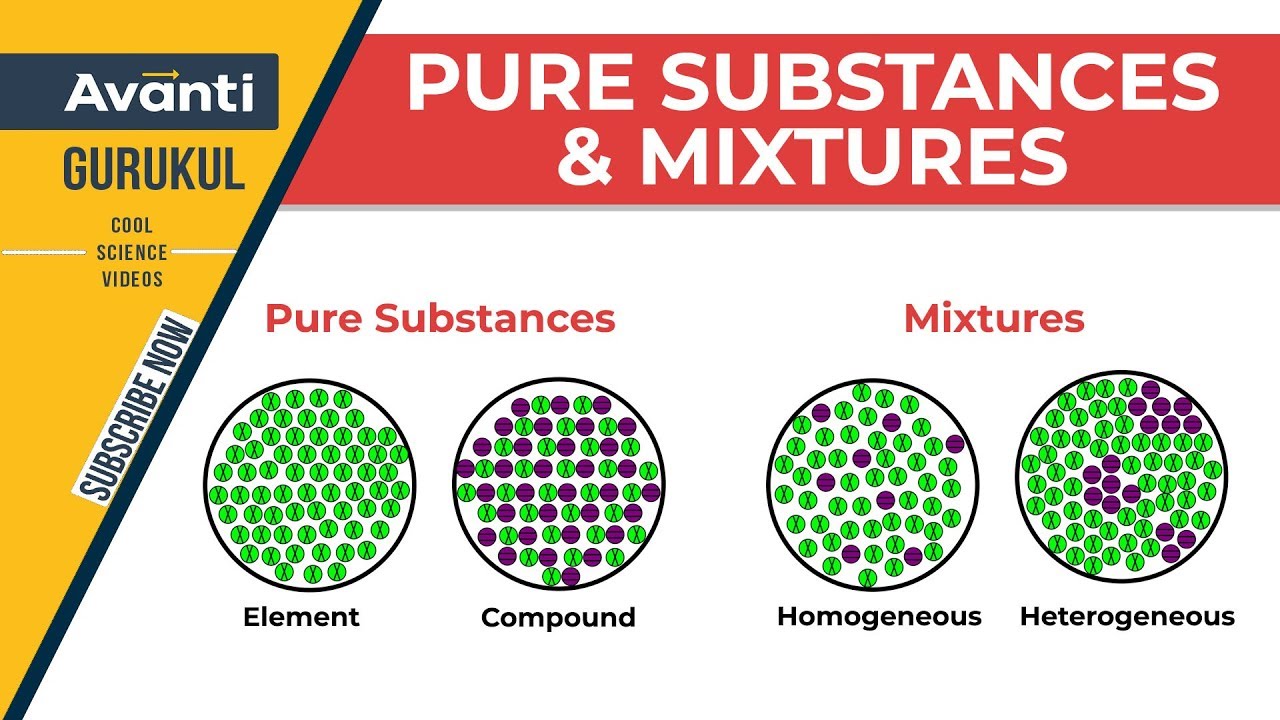
 00 Pure Substances Vs Mixtures Presentation
00 Pure Substances Vs Mixtures Presentation
Compound pure substance mixture and an element.
Pure substance or mixture. That is it is matter that appears uniform in appearance and composition no matter how small the sample size. A pure substance can also be defined as a form of matter that has both definite. A pure substance consists only of one element or one compound a mixture consists of two or more different substances not chemically joined together The components of a mixture can usually be.
A mixture can be separated into two or more pure substances. A pure substance is a substance made up of one component whereas a mixture is a substance that is made up of the combination of two or more components. A Pure Substance is matter which cannot be separated into its basic components by using a physical or a chemical process.
Examples of mixtures are air and alloys. In chemistry a pure substance consists of only one type of atom molecule or compound. Water is an example of a pure substance on the other hand salt mixed in water is an example of a mixture.
An element is a substance that consists of only one type or kind of atom. In the more general sense a pure substance is any homogeneous mixture. Pure Substances have sharp melting and boiling point and have standard melting or boiling point contrary to this boiling and melting points of mixtures varies by the proportion of constituents.
But there are exceptions to this definition. Pure substances cannot be separated into any other kinds of matter while a mixture is a combination of two or more pure substances. Pure substances are further classified as elements and compounds.
Explain the differences between the following. 1 Pure substance The substances that contain only one type of particle and they are free from any mixture are known as pure substances. By their chemical composition pure substances get divided into two types elements and compounds.
A chemical substance may well be defined as any material with a definite chemical composition in an introductory general chemistry textbook. The physical and chemical properties of pure substances are non-changing if it is on its own without disturbing. Examples of pure substances include iron steel and water.
If not you have a mixture. A pure substance consists only of one element or one compound a mixture consists of two or more different substances not chemically joined together The substances in a mixture can be elements or. Pure substances are substances that are made up of only one kind of particles and has a fixed or constant structure.
Homogeneous and heterogeneous mixtures B. If so you have a pure substance. Classification of Matter - YouTube All matter is made of pure substances and mixtures.
Add to my workbooks 11 Download file pdf Embed in my website or blog. 22 A collection of substances is called a mixture. Learn with flashcards games and more for free.
A chemical substance is a kind of matter with a definite composition and set of properties. Pure substances have the potential to form predictable products from chemical reactions. A pure substance can be either an element or a compound but the composition of a pure substance doesnt vary.
In this video Ill go over how to tell the difference between them through a short explanation and then. Mixture or Pure Substance. A pure substance is made up solely of that substance and cant be separated into any other substances.
Mole The mole is a unit of measurement that denotes an amount of substance also called chemical amount. A pure substance has constant physical and chemical properties while mixtures have varying physical and chemical properties ie boiling point and melting point. Air is a homogeneous mixture that is often considered to be a pure substance.
Classify substances into types of mixtures or pure substances ID. While pure substances have clearly defined physical and chemical properties mixtures have different properties depending on the proportions of the pure substances in each mixture and on the location in the mixture. A Mixture is made up of a combination of two or more substances that are not united using a chemical reaction.
Pure substances cannot be separated into other substances. 23 Mole and amount of substance Main article. A pure substance is in the purest form and has no impurities in it while mixture has impurities or is made up of two or more than substances.
According to this definition a chemical substance can either be a pure chemical element or a pure chemical compound. A pure substance also referred to as a chemical substance is a material that has a constant composition is homogenous and has consistent properties throughout the sample. The chemical and the physical properties of the pure substance remain the same throughout but they change in a mixture.
Some examples are carbon iron water sugar salt nitrogen gas and oxygen gas. Gold silver iron and aluminium are pure substances to name a few. Classifying Matter Other contents.
Elements are pure substances. Properties Of Homogeneous Mixtures.
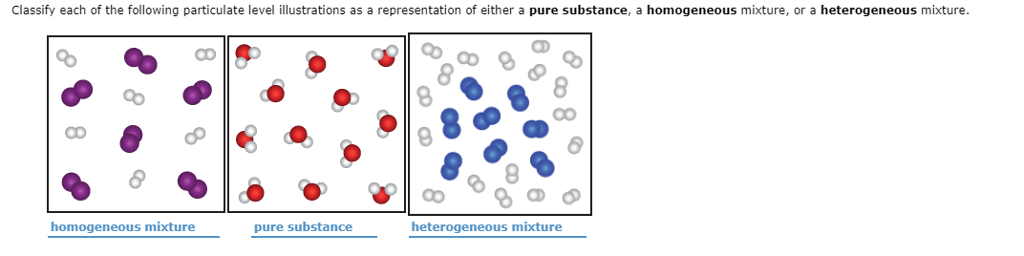 Solved Classify Each Of The Following Particulate Level I Chegg Com
Solved Classify Each Of The Following Particulate Level I Chegg Com
A homogeneous mixture is a mixture of substances blended so thoroughly that you cannot see individual substances.

Is a homogeneous mixture a pure substance. A heterogeneous mixture consists of two or more phases. Start studying Pure substance heterogeneous mixture or homogeneous mixture. Air is a homogeneous mixture because air is a thoroughly Also is oxygen a pure substance or compound.
When oil and water are combined they do not mix evenly but instead form two separate layers. Mixtures can be mainly divided into two as homogeneous mixtures and heterogeneous mixtures. For instance in pure water no other component is present except water molecules H 2 O.
A pure substance is a material made of only one element or type of atom. Homogeneous mixtures can be solid liquid gas or plasma mixtures. Every sample of the mixture will show the same amounts of each substance.
Therefore pure water is. Homogeneous means the same or uniform throughout. Chemical and physical properties may vary.
Heterogeneous Mixtures possess different compositions and properties in various parts. And these water molecules are made when fixed amounts of hydrogen and oxygen atoms react. Therefore pure substance is homogenous.
Mixtures are physical combinations of pure substances that have no definite or constant composition the composition of a mixture varies according to who prepares the mixture. Pure substances can be categorised as gas liquid and solid. However MANY mixtures are homogeneous but NOT a pure substance.
If all portions of a material are in the same state have no visible boundaries and are uniform throughout then the material is homogeneous. It may contain a number of the same element bonded together but it is still considered a pure. You cannot differentiate salt from water since they are seen by the naked eye as a single.
Further the properties of it are also uniform throughout the sample. Mixtures are categorised as homogeneous and heterogeneous. Is water a heterogeneous mixture.
Air is not a pure substance because it is a homogeneous mixture of different substances. The difference between a homogeneous mixture and a pure substance is that a pure substance has a fixed composition and cannot be separated because it is chemically bonded and a homogeneous mixture. Is air homogeneous or heterogeneous.
Examples include pure water H 2 gas gold. 1A pure substance is a form of matter that has a fixed chemical composition and a distinct characteristic while a homogeneous mixture is a mixture of two or more compounds with compositions that are uniform or mixed together in such a way that they are indistinguishable from each other. Mixtures that appear to be homogeneous are often found to be heterogeneous after microscopic examination.
Homogeneous mixture is composed of two or more chemical substances. Through definition a single-phase consists of a pure substance or a homogeneous mixture. Often it is easy to confuse a homogeneous mixture with a pure substance because they are both uniform.
The material is no longer a pure substance if it has been mixed with another pure substanceTwo pure substances mixed together are known as a mixturePure hydrogen is a pure substanceSo is pure honey even though it consists of many different types of molecules. Pure substance cannot be separated into two or more substances by any mechanical or physical method. Individual properties of components are retained.
Learn vocabulary terms and more with flashcards games and other study tools. Homogeneous mixture is a mixture that is made up of one phase only such as salt dissolved in water. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture.
When a homogeneous substance consists of the same type of molecule with fixed and uniform composition throughout then the substance is a pure substance. Therefore a homogeneous substance can either be a mixture or a pure substance. So all pure substances which include elements compounds are homogeneous.
Each of the layers is called a phase. It has a uniform composition throughout the sample. By definition a pure substance or a homogeneous mixture consists of a single phase.
The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample. Homogeneous means the substance looks the same throughout. There are two or more phases of a heterogeneous mixture.
Water is a pure chemical compound dihydrogen monoxide H2O. Chemical and physical properties are constant. It means the properties wont be uniform throughout the mixture.
A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform.