First define the percentage solution. It is a measure of solute concentration in a solution.
GMW Na 2299 Cl 3545 5844 X 855844.

Molarity from mass percent. Heres the equation we use to convert the percentage concentration to molarity. The molar mass of a compound sometimes to referred to as molecular weight is the cumulative atomic weight of all the atomselements in the compound. For example 1 mole of water H 2 O has a molar mass of roughly 180 grams.
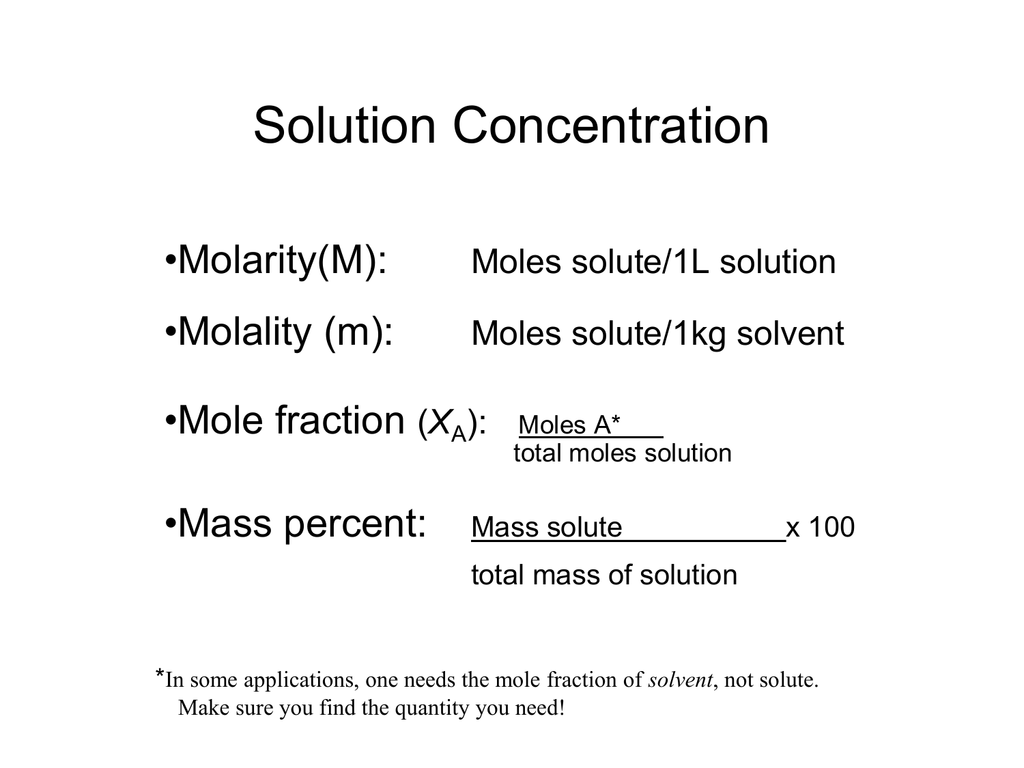
Molarity Percentage concentration Density Molar mass 100 The units required for this calculation are. 1600 mol 630119 gmol 100819 g 100819 g 1432 g 7040. Weight percent Mass of Xmass of X mass of X-1 Mass of X-n 100.
Mathematical manipulation of molality is the same as with molarity. It is defined as follows. The formula of the mass per cent is given as.
The molar mass of atoms of an element is given by the relative atomic mass of the element multiplied by the molar mass constant M u 1000 000 10 3 kgmol 1000000 gmol. Molar mass of Mn 549 g. Use the amount of substance of the solute divided by the volume to get molarity.
To dilute a solution of known molarity please use the Solution Dilution Calculator. Molarity - moldm³ M molL. ML 1432 g.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. X 85 gGMW x 10. You may have seen this symbol on the back of medicines and tablets.
It is also known as weight percent and is represented by ww. Percentage concentration - Density - gL gdm³. Let us now look at some solved examples of molarity to know in detail about what is molarity in chemistry.
085 wv solution 085 g100 mL. Mass percent fracmass of solutemass of solution 100. The mass molarity calculator tool calculates the mass of compound required to achieve a specific molar concentration and volume.
There are many different ways to express the concentration of solutions like molarity molality normality formality volume percentage weight percentage and part per million. Molar mass of K 391 g. Finding Mass Percentage and using it to determine an Empirical Formula.
It is one of the most commonly used units of representing concentration. Next calculate the mass-volume percent solution. Mass of X in g X in molL molar mass of X in gmolvolume in L of X Do the same for compound X-1 X-2 and however many compounds you have unless you know the mass of the total mixture.
2 Determine the mass percent just the nitric acid. Once the number of grams of solute per liter is known the molarity can be calculated. The molarity calculator tool provides lab-ready directions describing how to prepare an acid or base solution of specified Molarity M or Normality N from a concentrated acid or base solution.
Use the density of the solution to get the total volume of the solution. For normal samples from earth with typical isotope composition the atomic weight can be approximated by the standard atomic weight or the conventional atomic weight. To dilute a solution of concentrated acid or base of known ww strength please use the Acid Base Molarity Calculator.
Make sure that you count the atoms for each element and calculate the molar mass of each of the atoms. How to convert molarity to percentage concentration. Molality is also known as molal concentration.
If you prefer to think only in terms of liters not milliliters then simply consider mass percent as kilograms divided by liters. Calculate the molality mass percent and mole fraction of nitric acid in the solution. 0007 Statement of problem0043 Assume mass 100 g0113 Convert mass percents to actual masses0147 Calculate molar mass of KBr0245 Convert mass.
Then use the weight percent of solute to determine the amount of substance of the solute. Another way to specify an amount is percentage composition by mass or mass percentage mm. To prepare a solution from a solid reagent please use the Mass Molarity Calculator.
Since molarity is defined in terms of gL convert 085 g100 mL to gL. Note that the convention in molarity is to divide moles by liters but the convention in mass percent is to divide grams by milliliters. Find the molar mass of each element using the periodic table of elements.
Molarity is relevant to our concept of chemistry and kinetics of reactions. It is the ratio of the mass of solute to the mass of solution multiplied by 100 to calculate mass percent. In this video we look at how to calculate the molarity of a solution when you are given the mass percent and density of that solution.
1 Assume 1000 L of the solution is present. Not too difficult once. Then add all the X masses together.
Molar mass of O 160 g The solute contains 4 O atoms so count the 16g 4 times. The solution is composed of two components.