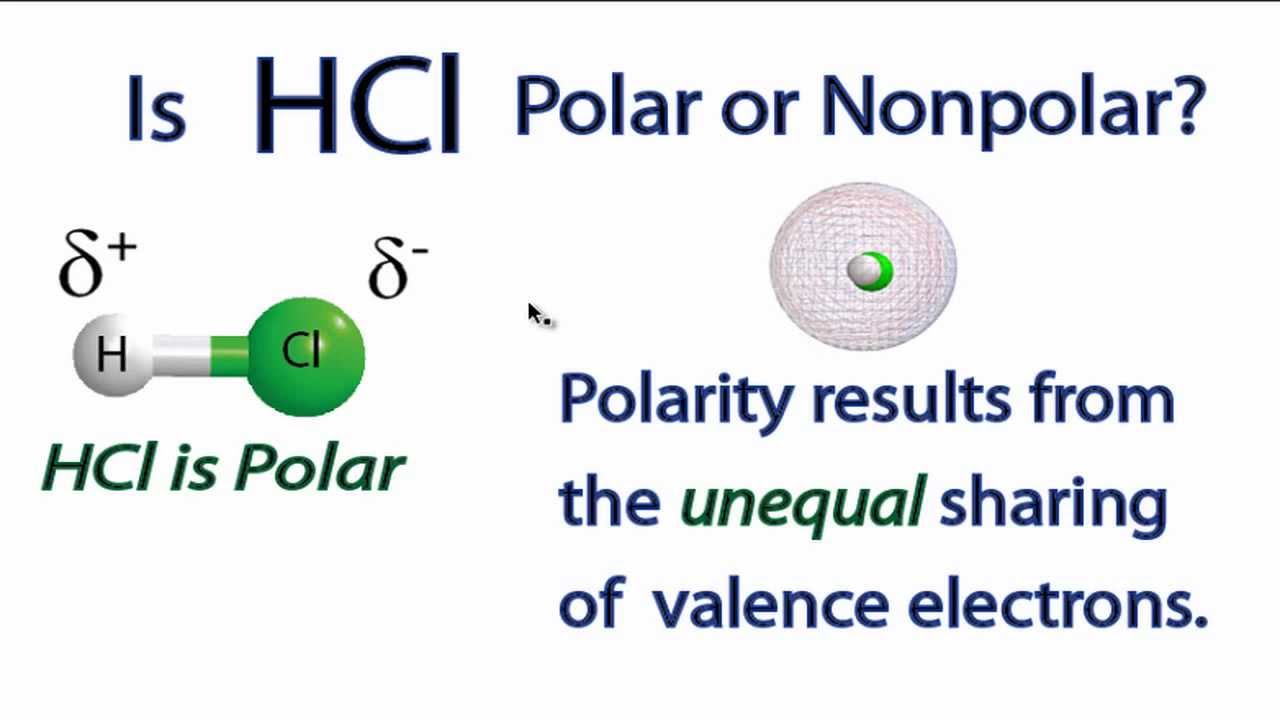
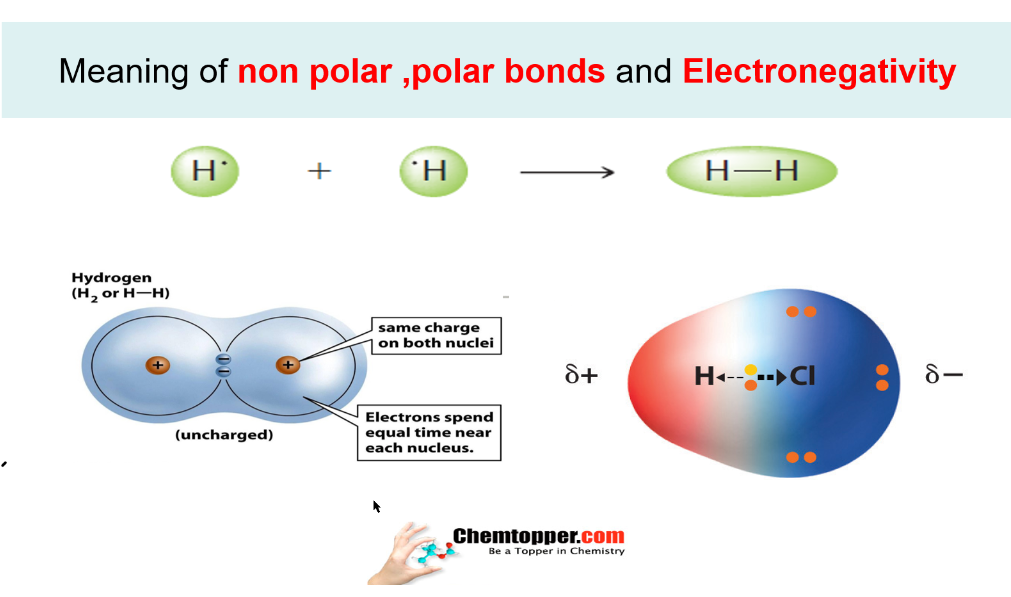
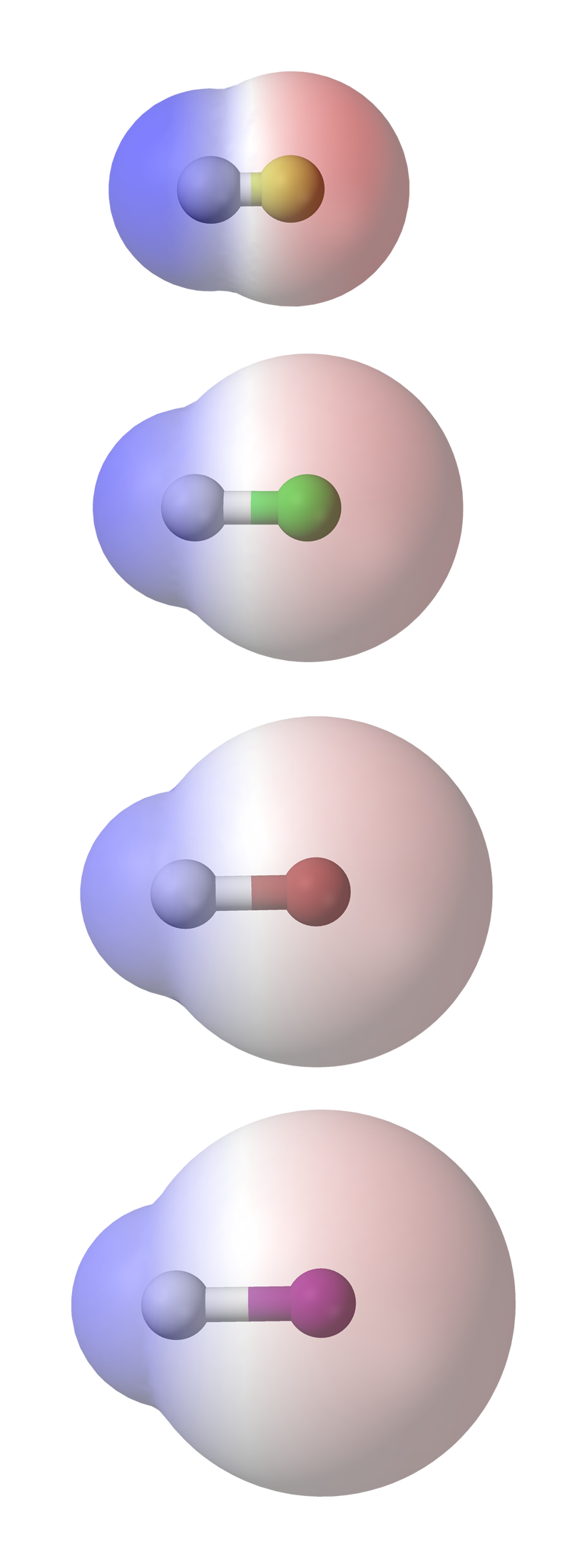There actually are simple HCL is a polar molecule as chlorine has a higher electronegativity than the hydrogen. Because of the force of attraction between oppositely charged particles there is a small dipole-dipole force of attraction between adjacent HCl molecules.
 Is Hcl Polar Or Nonpolar Youtube
Is Hcl Polar Or Nonpolar Youtube
Is hcl polar or nonpolar.
Is hcl polar or nonpolar. HCL is a polar molecule as chlorine has a higher electronegativity than the hydrogen. Question Is HCl hydrogen chloride polar or nonpolar. Is Nacl Polar Or Nonpolar Covalent Bond Indeed recently has been sought by users around us maybe one of you.
HCL is neither polar or non-polar. HCl is a polar molecule due to the large electronegativity difference between Chlorine 316 and hydrogen 220. A hydronium ion is considered polar.
Answer HCl hydrogen chloride is Polar. In these molecules the geometrical structure is also symmetric. A polar molecule consists of atoms having either the difference in electronegativities is what makes a bond polar or nonpolar.
Now consider hydrogen chloride hcl. Hcl polar or nonpolar atom closest to negative side. The only truly non-polar bond would be between atoms that have identical electronegativity.
The electrons will therefore spend most of their time hanging around the Cl rather than the H. Home Uncategorised is hcl polar or nonpolar. Ionic bond covalent bond james bond so many bonds.
Is hcl polar covalent. For example if the molecule were HCI and you decided the hydrogen atom was closest to the negative side of the molecule youd enter H in the last column of the table. 1 min ago Uncategorised.
HCl also known as Hydrogen Chloride is a gas at STP and is a polar molecule. Learn to determine if HCN is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and then use. HCl hydrochloric acid is a polar molecule because the chlorine is more electronegative than hydrogen due to which it attracts the bonded electron pair slightly nearer to it and gains a partial negative charge and hydrogen gains a partial positive charge.
In HCl the hydrogen atom is partially positively charged while the chlorine atom is partially negatively charged. There actually are simple HCL is a polar molecule as chlorine has a higher electronegativity than the hydrogen. Thus it attracts electrons to spend more time at its end giving it a negative charge and hydrogen a positive charge.
However in carbon dioxide polar covalent bonds CO are present but dipole. The two main classes of molecules are polar molecules and nonpolar moleculesSome molecules are clearly polar or nonpolar while others fall somewhere on the spectrum between two classes. Question Is ICl3 polar or nonpolar.
The dipole moment of HCl turns out to be 103 D. The Cl will have a resulting partial negative charge and the H will have a partial positive. HCl molecules for example have a dipole moment because the hydrogen atom has a slight positive charge and the chlorine atom has a slight negative charge.
The electronegativity of na is 093 and cl is 316. Water HCl and ammonia are polar molecule because they contain polar bonds and the net dipole moment is non-zero. This causes a region of partial positive charge on the hydrogen atom and a region of partial negative charge on the chlorine halide.
The hydrogen will sustain a slight positive. Hydrogen chloride HCl Polar or Nonpolar On the basis of characteristics HCl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it pulls shares pair of electrons from H atom as a result formation of partial positive charge on hydrogen and negative charge on chlorine atom. Posted February 11 2021.
If you were to dissolve HCl in water the aqueous solution will contain discreet hydronium ions H3O and chloride ions Cl-. The dominate intermolecular force in ICl is dipole- dipole whereas in Br2 it is London. Is HCl Polar or Nonpolar.
 Is Hcl Polar Or Nonpolar Techiescientist
Is Hcl Polar Or Nonpolar Techiescientist
 3 4 Bond Polarity Chemistry Libretexts
3 4 Bond Polarity Chemistry Libretexts
Why Is Hydrogen Chloride Polar Quora
 Covalent Bonds Chemistry Libretexts
Covalent Bonds Chemistry Libretexts
 Polar Covalent Bond An Overview Sciencedirect Topics
Polar Covalent Bond An Overview Sciencedirect Topics
 6 4 Polarity Of Molecules Introductory Chemistry
6 4 Polarity Of Molecules Introductory Chemistry
 Videoquiz Definition Electronegativity Polar Dipole Electron Affinity
Videoquiz Definition Electronegativity Polar Dipole Electron Affinity
 1 Polarity Polar Bonds Bonds Between Atoms Polar Molecules Polarity Between Molecules Occurs When Polar Bonds Create A Dipole Moment Ppt Download
1 Polarity Polar Bonds Bonds Between Atoms Polar Molecules Polarity Between Molecules Occurs When Polar Bonds Create A Dipole Moment Ppt Download
 Topic Polarity In Covalent Bonds Do Now What Is The Difference Between A Polar Molecule And Nonpolar Molecule Ppt Download
Topic Polarity In Covalent Bonds Do Now What Is The Difference Between A Polar Molecule And Nonpolar Molecule Ppt Download