Pure substances A pure substance has a definite and constant composition like salt or sugar. Is _____ a pure substance or a mixture.
 00 Pure Substances Vs Mixtures Presentation
00 Pure Substances Vs Mixtures Presentation
Compound pure substance mixture and an element.
Pure substance or mixture. That is it is matter that appears uniform in appearance and composition no matter how small the sample size. A pure substance can also be defined as a form of matter that has both definite. A pure substance consists only of one element or one compound a mixture consists of two or more different substances not chemically joined together The components of a mixture can usually be.
A mixture can be separated into two or more pure substances. A pure substance is a substance made up of one component whereas a mixture is a substance that is made up of the combination of two or more components. A Pure Substance is matter which cannot be separated into its basic components by using a physical or a chemical process.
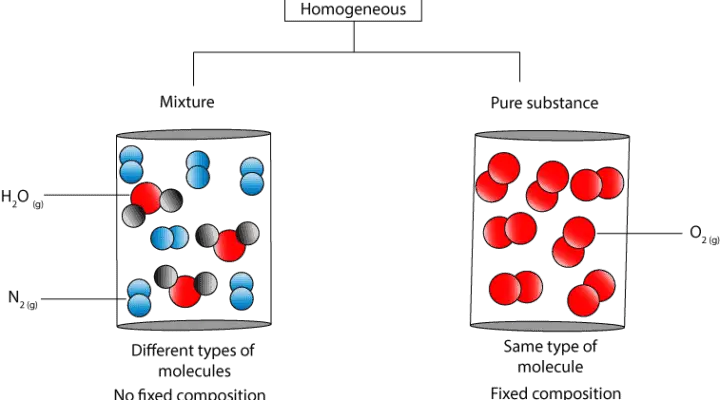
Examples of mixtures are air and alloys. In chemistry a pure substance consists of only one type of atom molecule or compound. Water is an example of a pure substance on the other hand salt mixed in water is an example of a mixture.
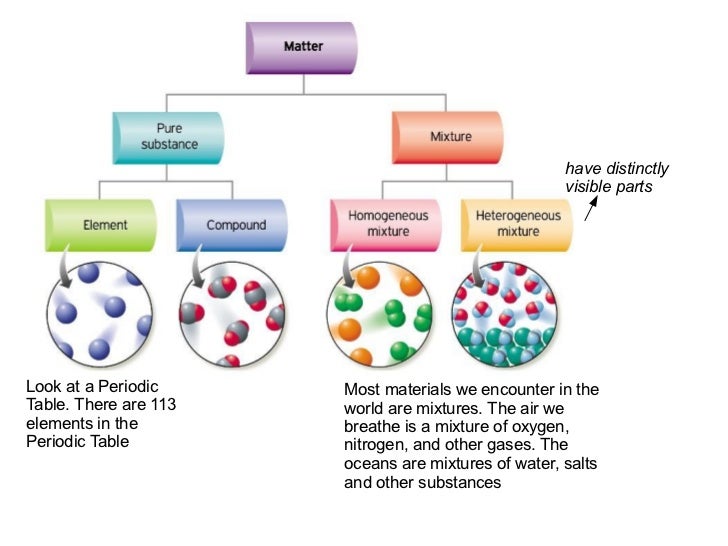
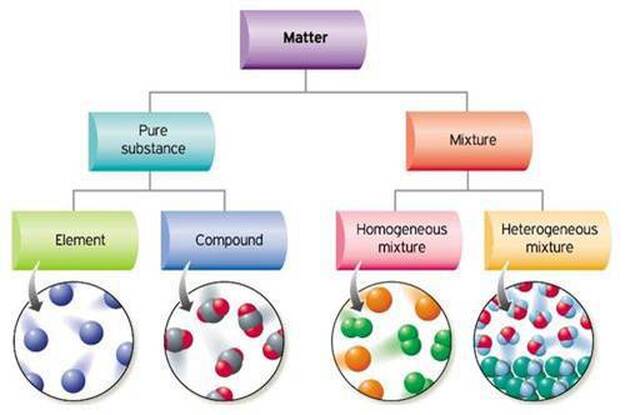
An element is a substance that consists of only one type or kind of atom. In the more general sense a pure substance is any homogeneous mixture. Pure Substances have sharp melting and boiling point and have standard melting or boiling point contrary to this boiling and melting points of mixtures varies by the proportion of constituents.
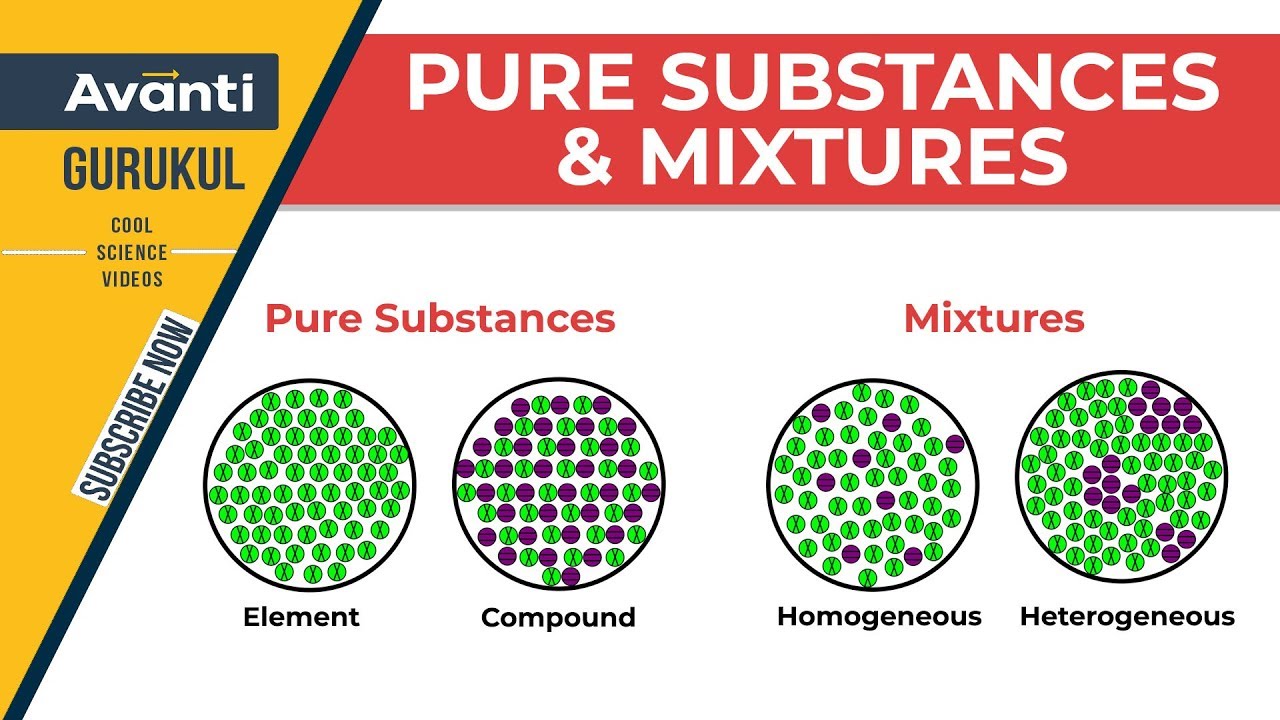
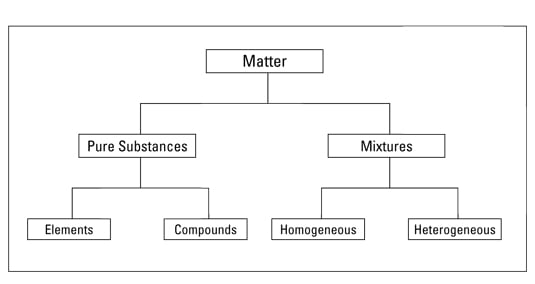
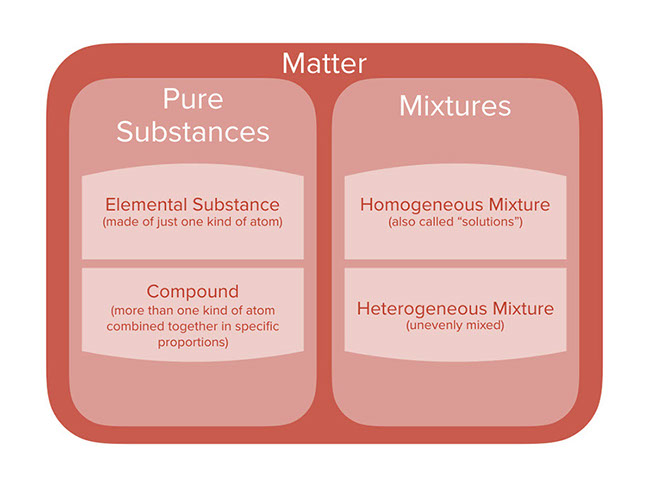
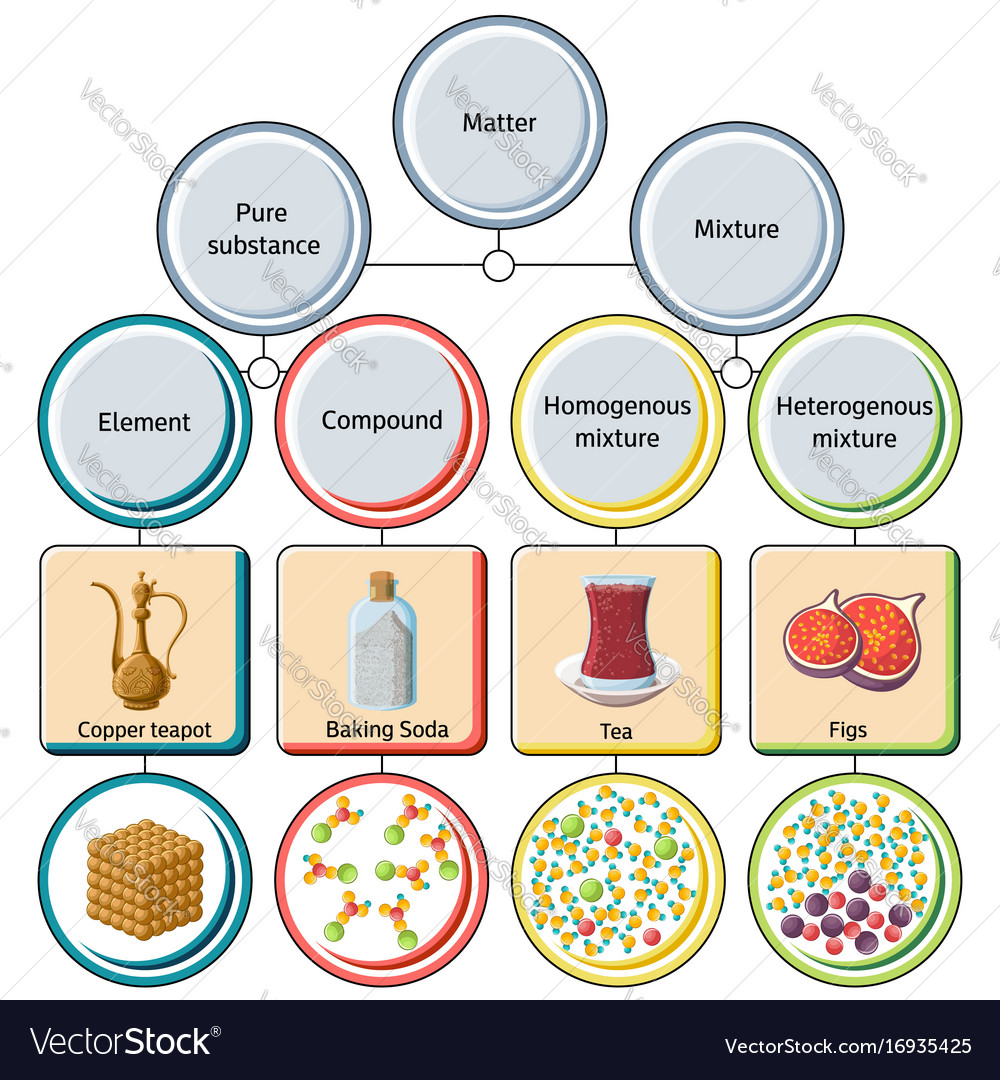
But there are exceptions to this definition. Pure substances cannot be separated into any other kinds of matter while a mixture is a combination of two or more pure substances. Pure substances are further classified as elements and compounds.
Explain the differences between the following. 1 Pure substance The substances that contain only one type of particle and they are free from any mixture are known as pure substances. By their chemical composition pure substances get divided into two types elements and compounds.
A chemical substance may well be defined as any material with a definite chemical composition in an introductory general chemistry textbook. The physical and chemical properties of pure substances are non-changing if it is on its own without disturbing. Examples of pure substances include iron steel and water.
If not you have a mixture. A pure substance consists only of one element or one compound a mixture consists of two or more different substances not chemically joined together The substances in a mixture can be elements or. Pure substances are substances that are made up of only one kind of particles and has a fixed or constant structure.
Homogeneous and heterogeneous mixtures B. If so you have a pure substance. Classification of Matter - YouTube All matter is made of pure substances and mixtures.
Add to my workbooks 11 Download file pdf Embed in my website or blog. 22 A collection of substances is called a mixture. Learn with flashcards games and more for free.
A chemical substance is a kind of matter with a definite composition and set of properties. Pure substances have the potential to form predictable products from chemical reactions. A pure substance can be either an element or a compound but the composition of a pure substance doesnt vary.
In this video Ill go over how to tell the difference between them through a short explanation and then. Mixture or Pure Substance. A pure substance is made up solely of that substance and cant be separated into any other substances.
Mole The mole is a unit of measurement that denotes an amount of substance also called chemical amount. A pure substance has constant physical and chemical properties while mixtures have varying physical and chemical properties ie boiling point and melting point. Air is a homogeneous mixture that is often considered to be a pure substance.
Classify substances into types of mixtures or pure substances ID. While pure substances have clearly defined physical and chemical properties mixtures have different properties depending on the proportions of the pure substances in each mixture and on the location in the mixture. A Mixture is made up of a combination of two or more substances that are not united using a chemical reaction.
Pure substances cannot be separated into other substances. 23 Mole and amount of substance Main article. A pure substance is in the purest form and has no impurities in it while mixture has impurities or is made up of two or more than substances.
According to this definition a chemical substance can either be a pure chemical element or a pure chemical compound. A pure substance also referred to as a chemical substance is a material that has a constant composition is homogenous and has consistent properties throughout the sample. The chemical and the physical properties of the pure substance remain the same throughout but they change in a mixture.
Some examples are carbon iron water sugar salt nitrogen gas and oxygen gas. Gold silver iron and aluminium are pure substances to name a few. Classifying Matter Other contents.
 How To Distinguish Pure Substances And Mixtures Dummies
How To Distinguish Pure Substances And Mixtures Dummies
 What Are The Types Of Pure Substances And Mixtures A Plus Topper
What Are The Types Of Pure Substances And Mixtures A Plus Topper
 Pure Substances Mixtures Is Matter Around Us Pure Youtube
Pure Substances Mixtures Is Matter Around Us Pure Youtube
 Lesson Categories Of Chemicals And Mixtures
Lesson Categories Of Chemicals And Mixtures
 Unit 3 Pure Substances And Mixtures San Francisco De Paula Science Department
Unit 3 Pure Substances And Mixtures San Francisco De Paula Science Department
 What Are Pure Substances And Mixtures Posters Teaching Resource Teach Starter
What Are Pure Substances And Mixtures Posters Teaching Resource Teach Starter
 What Are The Types Of Pure Substances Compounds Elements Videos
What Are The Types Of Pure Substances Compounds Elements Videos
Pure Substances And Mixtures Bioprofe
 Pure Substances And Mixtures Diagram Royalty Free Vector
Pure Substances And Mixtures Diagram Royalty Free Vector
 Pure Substance Or Mixture Worksheet
Pure Substance Or Mixture Worksheet
 If A Substance Is Homogeneous Is It A Pure Substance
If A Substance Is Homogeneous Is It A Pure Substance


