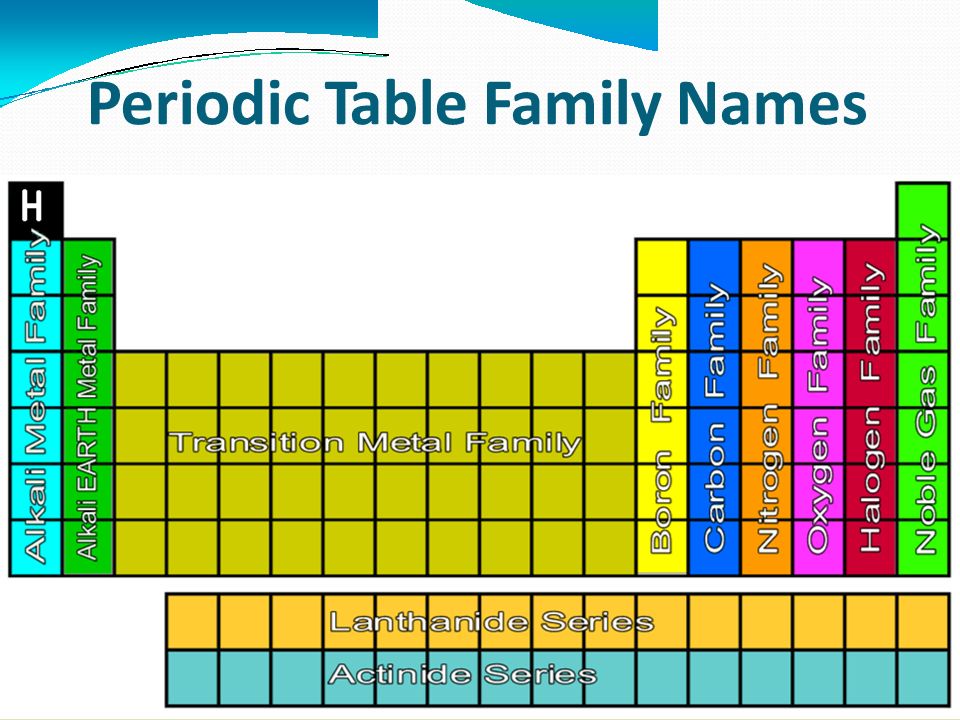
The figure below lists some important families that are given special names. The halogen family consists of fluorine chlorine bromine iodine and astatine.
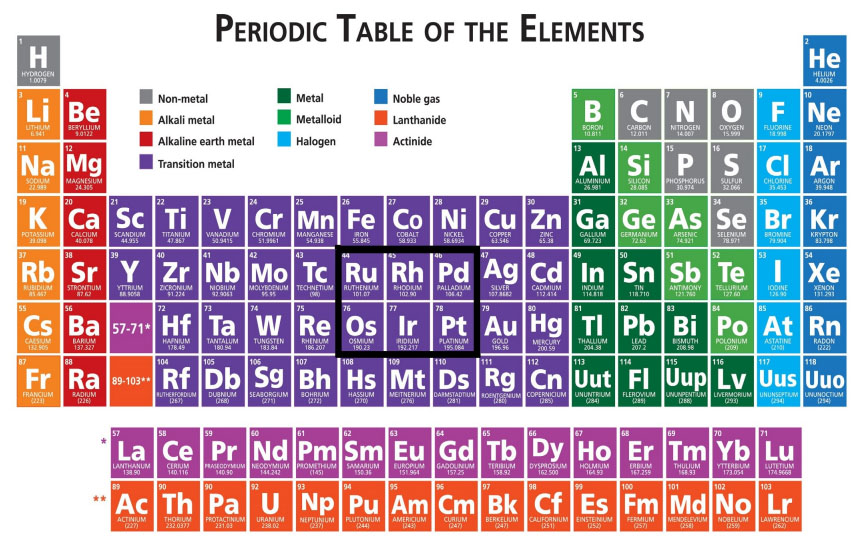 Platinum And 150 Years Of The Periodic Table
Platinum And 150 Years Of The Periodic Table
There are 18 numbered groups in the periodic table.
How many families are in the periodic table. There are more than 100 elements which are arranged in the periodic table and each is represented by a symbol. And always remember Al and Po arent non-metals even though theyre. The f-block columns between groups 2 and 3 are not numbered.
The families are the names representing each group. You can examine four families on the periodic table and look at the electron configurations for a few elements in each family. Group 2A of the periodic table.
The current standard table contains 118 confirmed elements as of 2019. The older IUPAC system used Roman numerals together with letters to distinguish between the left A and right B side of the periodic table. There are 9 different families and these are the.
The Royal Society of Chemistrys interactive periodic table features history alchemy podcasts videos and data trends across the periodic table. When the elements are thus arranged there is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties. Element families are elements that have the same number of valence electrons.
It took time for the periodic table of elements to develop into its current form and many of its early iterations such as this one called Mendeleevs Flower would be unrecognizable today. Periodic table in chemistry the organized array of all the chemical elements in order of increasing atomic number. Three systems have been used to number families and groups.
Group 7A of the periodic table. The section in the. There are multiple ways of grouping the elements but they are commonly divided into metals non-metals metalloids.
A vertical column in the periodic table. Use the buttons above to change your view of the periodic table and view Murray Robertsons stunning Visual Elements artwork. Group 1A of the periodic table.
In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elements. Groups are also known as Families. In the periodic table of chemical elements there is a group also known as a family is a column of elements.
Most element families are a single column of the periodic table although the transition elements consist of several columns plus the elements located below the main body of the table. This Periodic Table shows that there is nine different families some example are the Non metals and Alkali Metals and so forth down the line as seen on the image to the side. One common substance.
You can use the term family and group inter-changeably so yes these are the 11 families groups in the periodic table. Families on the periodic table include in addition to noble gases and halogens the alkali metals alkaline earth metals transition metals lanthanides and actinides. They all have seven valence electrons which make them highly reactive for covalentbonds.
Group 8A of the periodic table. The nonmetals form a loosely defined cross-family grouping as do the metalloids. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms ie the same core charge because most chemical properties are dominated by the orbital location of the outermost electron.
The 18 vertical columns of the table are called Groups. Columns of the periodic table typically mark groups or families. The IA family is made up of the alkali metals.
Are located in the second column from the right side of the periodic table group 17. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right. In reactions these elements all tend to lose a single electron.
Click the tabs at the top to explore each section. The properties of the chemical elements are often summarized using the periodic table which powerfully and elegantly organizes the elements by increasing atomic number into rows in which the columns share recurring periodic physical and chemical properties. Halogens form ionic bonds with all kinds of elements.
There are also more specific groups like alkali metals transition metals rare metals alkaline earth halogens and noble gases. The Periodic table can be divided into nine families of elements each having similar properties. A section of the periodic table showing metals and non-metals The main groups are numbered from 1 to 7 going from left to right and the last group on the right is group 0.
But the periodic table is also an important aspect of science education. An example of an element family is the nitrogen group or pnictogens. Groups 3 to 12 of the periodic table.
Recognizing Families on the Periodic Table. A section of the periodic table is shown below which illustrates how the elements are.
/GettyImages-470784875-e7ea7f0da3d74a639d8e6a290afa000b.jpg) What Is A Group Or Family On The Periodic Table
What Is A Group Or Family On The Periodic Table
 Color And Learn About The Periodic Table Layers Of Learning Periodic Table Of The Elements Teaching Science Physical Science Middle School
Color And Learn About The Periodic Table Layers Of Learning Periodic Table Of The Elements Teaching Science Physical Science Middle School
Group Periodic Table Wikipedia

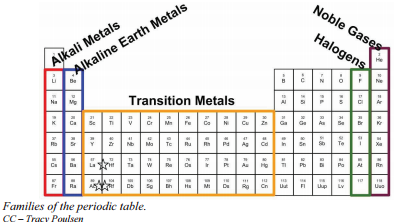 2 3 Families And Periods Of The Periodic Table Chemistry Libretexts
2 3 Families And Periods Of The Periodic Table Chemistry Libretexts
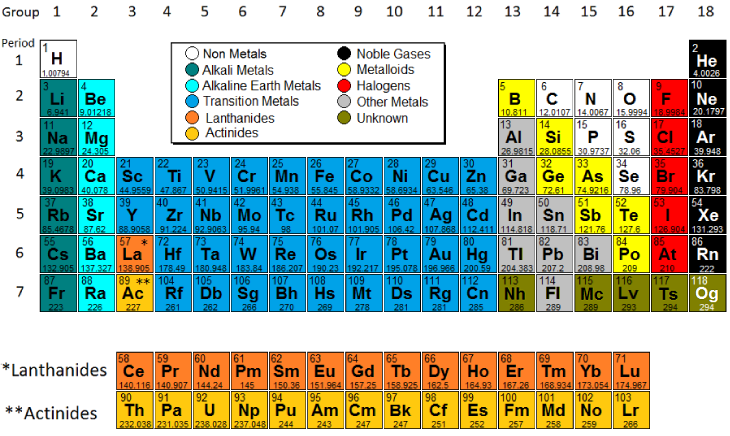 Periodic Table Model Science Software
Periodic Table Model Science Software
:max_bytes(150000):strip_icc()/Elements-58f7944e5f9b581d59396ae7.jpg) Element Families Of The Periodic Table
Element Families Of The Periodic Table
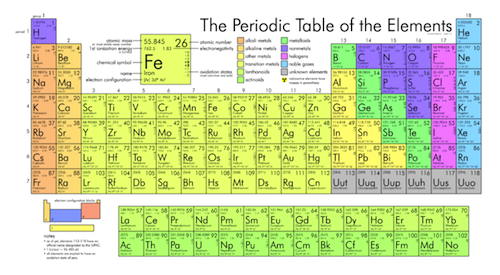 The Periodic Table Help Chemistry Basics Study Guide Shmoop
The Periodic Table Help Chemistry Basics Study Guide Shmoop
The Periodic Table Families The Periodic Table
/periodic-table-165930186-590f2d703df78c92832fe141.jpg) Family Definition Chemistry Glossary
Family Definition Chemistry Glossary
 Families Of The Periodic Table Youtube
Families Of The Periodic Table Youtube