Metal blue nonmetal yellow or metalloid red. Three metals iron cobalt and nickel are magnetic.
 Understanding The Periodic Table Through The Lens Of The Volatile Group I Metals
Understanding The Periodic Table Through The Lens Of The Volatile Group I Metals
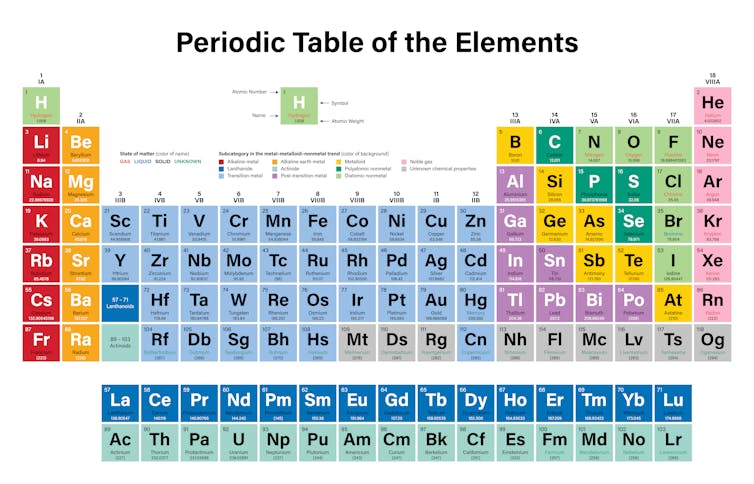
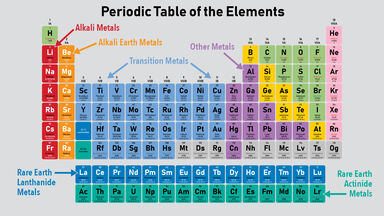
The metals are subdivided into separate groups such as basic metals transition metals alkali metals alkaline earth metals rare earth lanthanides and actinides.
Metal in periodic table. The archetypal transition metals and the physically and chemically weak post-transition metals. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides. Metal elements are on the left of a stepped line starting at B-Al-Si non-metal elements are on the right of the stepped line.
Examples of metals are gold aluminium copper iron lead silver platinum uranium and zinc. Except for Germanium Ge and Antimony Sb all the elements to the left of that line can be classified as metals. These metals will have 1 2 or 3 electrons in the outer shell.
This periodic table groups elements according to type. Nearly 75 of all the elements in the Periodic Table are classified as metals. Most elements are metals.
Metals In The Periodic Table So because most elements of the Table are metals it makes sense to begin by looking at them. Heavy metals up to the vicinity of iron in the periodic table are largely made via stellar nucleosynthesis. If you look at the Periodic table you will find that the metal elements are located between atomic number 5 Boron B all the way to atomic number 84 Polonium Po.
Although separate on the periodic table lanthanides and actinides are really specific types of transition metals. The outer shell of a metal is therefore less than half full of. At room temperature all of the metals are solids except for mercury which is a liquid.
The metal elements are on the left of a stepped line. From left to right in the periodic table these categories include the highly reactive alkali metals. Many scientists describe a transition metal as any element in the d-block of the periodic table which includes groups 3 to 12 on the periodic table.
In the Periodic Table metals are separated into the groups detailed in the following list. There may be many unforeseen applications that arise from learning about the. They are grouped together in the middle to the left-hand side of the periodic table.
The highlighted elements are considered the metal elements. The IUPAC definition defines a transition metal as an element whose atom has a partially filled d sub-shell or which can give rise to cations with an incomplete d sub-shell. One ounce of gold can be easily converted to 40 meters long wire.
In the periodic table you can see a stair-stepped line starting at Boron B atomic number 5 and going all the way down to Polonium Po atomic number 84. The less reactive alkaline earth metals lanthanides and radioactive actinides. In this process lighter elements from hydrogen to silicon undergo successive fusion reactions inside stars releasing light and heat and forming heavier elements with higher atomic numbers.
In the periodic table. Hence Gold is the most malleable metal in the. The majority of elements on the periodic table are metals.
This group includes alkali metals alkaline earth metals transition metals basic metals lanthanides rare earth elements and actinides. All of the metals are grouped. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right.
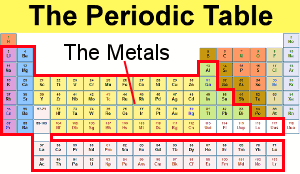
Most elements can be considered metals. The periodic table shows that metals are found in groups 1 2 and 3. Metals and non-metals There are many divisions in the Periodic Table but one of the most important is between the metals and the non-metals.
Gold and silver are the most malleable metal present in the periodic table. 75 of all the elements on the periodic table are metals. Steel is a mixture of elements but it is mostly iron so it is also magnetic.
Such chemistry also explains why mercury metal is a liquid at room temperature despite periodic table predictions. The other metal elements are not magnetic.
 Metals Chemistry For Non Majors
Metals Chemistry For Non Majors
 Metals And Non Metals Of The Periodic Table
Metals And Non Metals Of The Periodic Table
 Metals Nonmetals And Metalloids On The Periodic Table Youtube
Metals Nonmetals And Metalloids On The Periodic Table Youtube
 Definition Of Metals Chemistry Dictionary
Definition Of Metals Chemistry Dictionary
Classification Of Elements In The Periodic Table Color Coding Yellow Download Scientific Diagram
 Basic Types Of Metals On The Periodic Table
Basic Types Of Metals On The Periodic Table
 Alkali Metal Definition Properties Facts Britannica
Alkali Metal Definition Properties Facts Britannica
Groups And Periods Of The Periodic Table Metals Nonmetals And Metalloids Elements And The Periodic Table
 What Makes A Metal Chemistry Periodic Table Periodic Table Of The Elements
What Makes A Metal Chemistry Periodic Table Periodic Table Of The Elements
The Periodic Table Chemistry Socratic



/Periodic-Table-Metals-56a12db33df78cf772682c44.png)