Metallic and Non metallic PropertiesHi FriendsIn this video we will learn about the metallic and non metallic properties of elements in periodic tableMetal. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides.
Classification Of Elements In The Periodic Table Color Coding Yellow Download Scientific Diagram
Three metals iron cobalt and nickel are magnetic.
Metallic elements in the periodic table. 95 - Atomic Mass. Metals tend to form positive ions and like charges repel so how do. They are grouped together in the middle to the left-hand side of the periodic table.
89 - Atomic number. The fact that the metallic elements are found on the left side of the periodic table offers an important clue to the nature of how they bond together to form solids. They can be described as a lattice of positive ions surrounded by a cloud of delocalized electrons.
The elements in group 3 have an ns 2 n 1d 1 configuration. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right. These elements all possess low electronegativities and readily form positive ions.
33 - Melting point. Atomic number - Name alphabetically. If you look at the Periodic table you will find that the metal elements are located between atomic number 5 Boron B all the way to atomic number 84 Polonium Po.
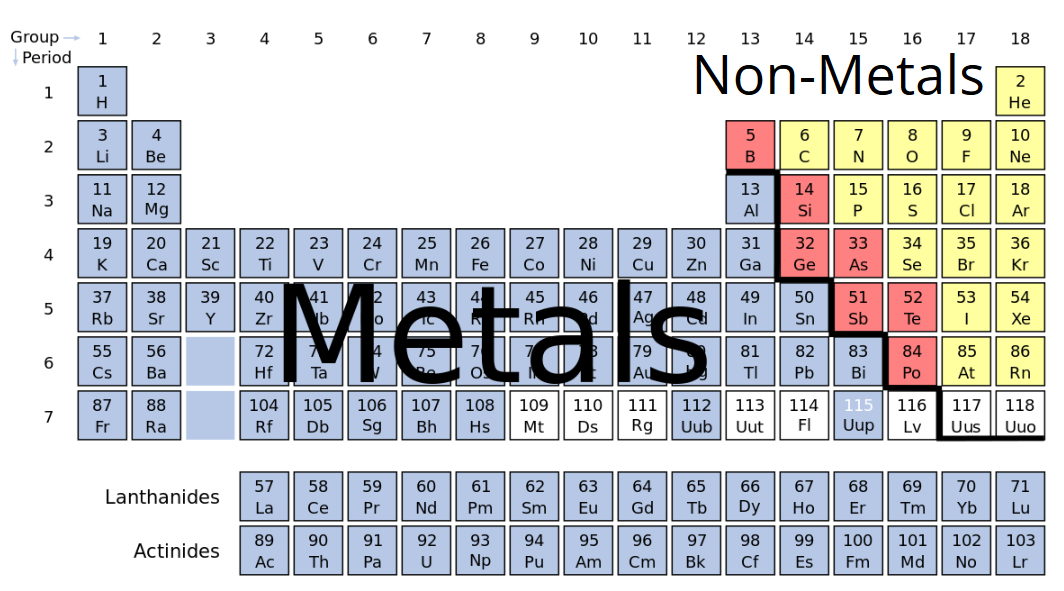
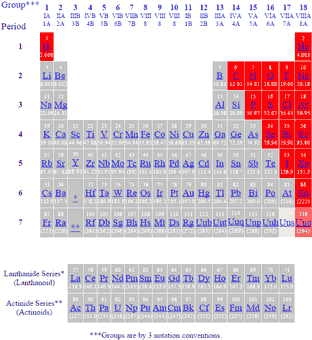
The nonmetals or non-metals are a group of elements located on the right side of the periodic table except for hydrogen which is on the top left. The various elements in the periodic table are divided into three parts. The Periodic Table Consists of a Continuum of Elements There is a simple way to distinguish metals from non-metals in the Periodic Table.
Elements with low electronegativity tend to have more metallic properties. Metals In The Periodic Table. The Periodic Table contains both metals and non-metals.
Metals form ionic bonds with non-metals. The chemical elements of the periodic chart sorted by. So because most elements of the Table are metals it makes sense to begin by looking at them.
Heres a list of all the elements on the periodic table that are metals. This entry was posted on September 3 2014 by Todd Helmenstine updated on April 9 2020 The highlighted elements are considered the metal elements. Metal elements are on the left of a stepped line starting at B-Al-Si non-metal elements are on the right of the stepped line Metals are on the left of the periodic table and.
Periodic Table Metals A metal is an element that readily loses electrons to form positive ions cations and has metallic bonds between metal atoms. In periodic table terms the noble metals correspond to the noble gases. The short list of chemically noble metals those elements upon which almost all chemists agree comprises ruthenium Ru rhodium Rh palladium Pd osmium Os iridium Ir platinum Pt and gold Au.
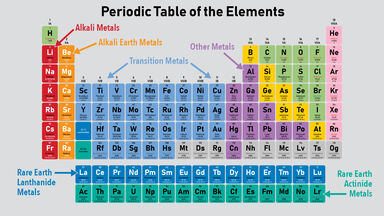
56 - Vanderwaals radius. This group includes alkali metals alkaline earth metals transition metals basic metals lanthanides rare earth elements and actinides. 85 - Boiling point.
The metals are one of the three groups of elements as. Noble metals in the periodic table Elements categorised as such. So the metallic properties of elements tends to decrease across a period and increase down a group.
Steel is a mixture of elements but it is mostly iron so it is also magnetic. Except for Germanium Ge and Antimony Sb all the elements to the left of that line can be classified as metals. In the periodic table the transition metals are present in eight groups 4 to 11 with some authors including some elements in groups 3 or 12.
Use your periodic table of elements to answer all the following questions. Although separate on the periodic table lanthanides and actinides are really specific types of transition metals. Identify an element that shares the characteristics below.
Metals-Metals are the group of certain elements that lose electrons. The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. Periodic Table Concepts CWHW Directions.
In the periodic table. The other metal elements are not magnetic. Most elements are metals.
Juan Ramos on November 24 2017 53 Comments. The more an element exhibits metallic properties the greater tendency it has to form ionic rather than covalent bonds with other less metallic atoms. Most elements can be considered metals.
Metals are present on the left side of the periodic table. These elements are distinctive in that they typically have low melting and boiling points dont conduct heat or electricity very well and tend to have high ionization energies and electronegativity values. In the periodic table you can see a stair-stepped line starting at Boron B atomic number 5 and going all the way down to Polonium Po atomic number 84.
 Metals Nonmetals And Metalloids On The Periodic Table Youtube
Metals Nonmetals And Metalloids On The Periodic Table Youtube
 Basic Types Of Metals On The Periodic Table
Basic Types Of Metals On The Periodic Table
 Periodic Table Of The Elements Non Metals
Periodic Table Of The Elements Non Metals
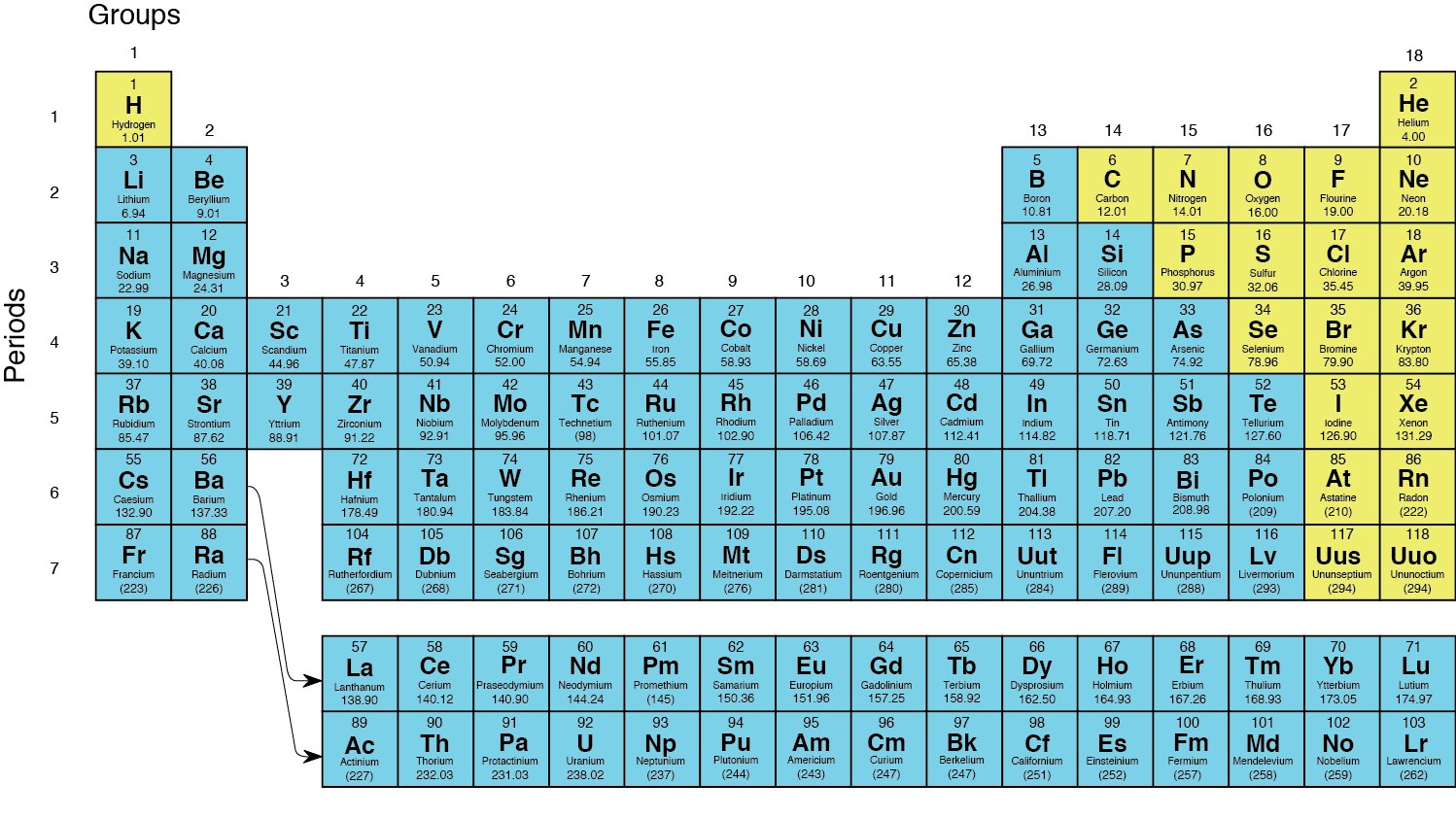
 P Strong Fig 2 12 Strong The Periodic Fig 2 12 The Periodic Table Of The Elements 2014 This Periodic Table Shows Metal Elements In Blue And Nonmetal Elements In Yellow Image By Byron Inouye
P Strong Fig 2 12 Strong The Periodic Fig 2 12 The Periodic Table Of The Elements 2014 This Periodic Table Shows Metal Elements In Blue And Nonmetal Elements In Yellow Image By Byron Inouye
 Alkali Metal Definition Properties Facts Britannica
Alkali Metal Definition Properties Facts Britannica
 Layers Of Learning Science Hands On Experiments Family Style Learning Science Elementary Chemistry Chemistry
Layers Of Learning Science Hands On Experiments Family Style Learning Science Elementary Chemistry Chemistry
/arc-anglerfish-arc2-prod-bonnier.s3.amazonaws.com/public/7LBTJXWW447EWOKW7PKFM2F5EY.jpg) Four New Elements Added To The Periodic Table Popular Science
Four New Elements Added To The Periodic Table Popular Science
:max_bytes(150000):strip_icc()/nonmetals-56a12d975f9b58b7d0bccfd3.png) List Of Elements That Are Nonmetals
List Of Elements That Are Nonmetals
 How Can We Identify Elements As Metal Or Non Metals Quora
How Can We Identify Elements As Metal Or Non Metals Quora
 Boron Based Atomic Clusters Mimic Rare Earth Metals
Boron Based Atomic Clusters Mimic Rare Earth Metals