Use the formula q mΔH v in which q heat energy m mass and ΔH v heat of vaporization. This is called fusion.
2 kg of ice at 2 0 o C is mixed with 5 kg of water at 2 0 o C in an insulating vessel having a negligible heat capacity.
How to find latent heat of vaporization. This means that to convert 1 g of water at 100 ºC to 1 g of steam at 100 ºC 2260 J of heat must be absorbed by the water. The latent heat is calculated at constant pressure isobaric process and the vaporization of a pure component occurs at constant temperature isothermal process and of course constant composition. Water has high specific heat.
And c specific heat terms in the temperature-change formula. Heat of Vaporization of Water. 50 g of water was used.
ΔH vap ΔU vap pΔV. Calculate the final mass of water in the vessel. Heat of Vaporization of Water H v 2260 J g.
Temperature-dependency of the heats of vaporization for water methanol benzene and acetone. Next determine the total heat. M is the mass and L is the latent heat.
In other words the heat of vaporization is the total amount of heat required to turn a particular quantity of liquid into vapour without any rise in the temperature of the fluid. Add heat to the liquid until the liquid starts to vaporize. Here L takes the place of the.
The latent heat we cant use the latent heat of vaporization. The three states of matter are solid liquid and gas. Heres the formula for heat transfer during phase changes where.
The Formula for Heat of Vaporization. Based on entropy and enthalpy of the process of vaporization the Heat of vaporization formula is. Calculate the latent heat of vaporisation of water.
This is the heat per kilogram needed to make the change between the solid and gas phases as when dry ice evaporates. First determine the mass of the liquid. Latent heat of vaporisation 10800 005 2160000 Jkg 2160 kJkg.
When heat is added to a system from the. This is a solid turning into a liquid. 5 k c a l k g 1 o C 1 respectively and the latent heat of fusion of.
L fracQ M Where Q amount of heat. Q mΔH f q 25 gx540 calg q 13500 cal Answer. Where ΔU vap is the difference in internal energy between the vapor phase and the liquid phase ΔU vap H vapor H liquid and pΔV is the work done against the ambient pressure.
The heat of vaporization is different for different substances but is a constant for each individual one. It is also known as the enthalpy of. The energy of a moving object measured in Joules.
This equation states that the heat Q that must be added or removed for an object of mass m to change phases. The unit of latent heat is Jkg-1 The value of latent heat is variable. The latent heat of vaporization of a substance is defined as the heat energy needed to vaporize the unit mass of a substance without change in its temperature.
This measurement describes the amount of energy it takes to raise the temperature of water 1 degree Celsius. Thats latent heat of fusion that we need and the latent heat of fusion for water is about 333000 joules per kilogram which gives you 999000 joules of heat in order to turn this ice at zero degree Celsius into water at zero degrees Celsius. When ice a solid melts it turns into water a liquid.
Conversely when 1 g of steam at 100 ºC condenses to give 1 g of water at 100 ºC 2260 J of heat will be released to the surroundings. The amount of heat consumed or released in a system at constant pressure. Measure the total mass of the liquid being vaporized.
In case of liquid to gas phase change this amount of energy is known as the enthalpy of vaporization symbol H vap. For water at its normal boiling point of 100 ºC the heat of vaporization is 2260 J g -1. J also known as the latent heat of vaporization or heat of evaporation.
Vaporization or Evaporation the transition of molecules from a liquid to a gaseous state. The latent heat of vaporization ΔH corresponds to the amount of energy that must be supplied to the system to convert a unit amount of substance from the liquid to the vapor phase under conditions of equilibrium between the two phases. The calculation of the heat of vaporization of pure components latent heat is very straightforward.
We denote Individual latent heat by L. It is given that the specific heats of water and ice are 1 k c a l k g 1 o C 1 and 0. 50 g 50 1000 005 kg.
The molecules on a surface are usually the first to undergo a phase change. The enthalpy of vaporization symbol Hvap also known as the latent heat of vaporization or heat of evaporation is the amount of energy enthalpy that must be added to a liquid substance to transform a quantity of that substance into a gas. If the solutions of vapour and liquid states are compared the kinetic energy of the steam is proved to be higher than the kinetic energy of the fluid.
The amount of energy required is the heat of vaporization. M mass of the substance. H_v frac q m.
This topic is usually explored in thermodynamics undergraduate courses. As an example see the figure which descibes phase transitions of water. The Formula for Latent Heat.
This transition thus always occurs at constant temperature and the corresponding vapor pressure p s. Q 25 gx2257 Jg q 56425 J Part II. Hence a more complete equation to calculate the heat of vaporization is.
Latent Heat Of Fusion And Vaporization Specific Heat Capacity Calorimetry Physics Youtube
Physics Latent Heat Angelica11a
The Open Door Web Site Ib Physics Practicals Thermal Physics To Measure The Specific Latent Heat Of Vaporisation Of Water
Solved Blundell 28 4 A Show That The Temperature Depen Chegg Com
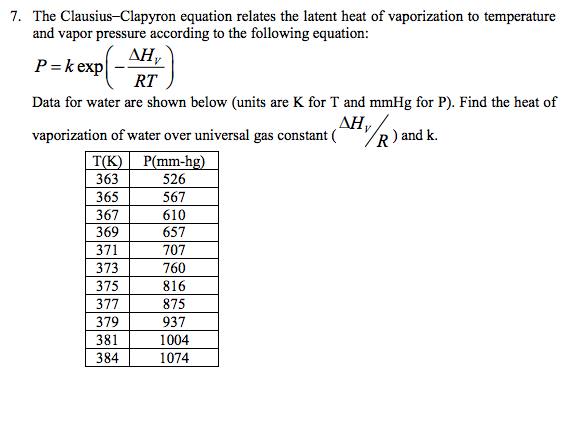
Solved The Clausius Clapyron Equation Relates The Latent Chegg Com
Heat And Temperature Heat Is A Form Of Energy And Is Measured In Joules J Temperature Is Different From Heat Temperature Is A Measure Of How Hot Or Ppt Video Online
Measuring The Specific Latent Heat Of Vaporisation Of Water Spm Physics Form 4 Form 5 Revision Notes
Measuring The Specific Latent Heat Of Vaporization Of Water Youtube
How To Calculate The Latent Heat Needed To Cause A Phase Change Dummies
Geo3020 4020 Lecture 2 I Energy Balance Ii Evapotranspiration Ppt Video Online Download
Specific Latent Heat Examples Solutions Videos Notes
Finding Latent Heat Of Vaporisation As A Function Of Temperature Physics Forums
ads
Slide Course
Search This Blog
Labels
- 1041
- 11th
- 12th
- 16th
- 2007
- 2010
- 2013
- 401k
- 75th
- 85th
- abbreviations
- abreviation
- acceleration
- account
- accounting
- accounts
- action
- activate
- active
- actual
- adding
- additional
- address
- adhesion
- adjectives
- administration
- adobe
- advisor
- aerate
- aero
- agent
- algebra
- alive
- always
- amendment
- amendments
- amps
- analysis
- anatomical
- android
- angle
- angles
- angular
- animate
- another
- anti
- anyone
- aperture
- apple
- archaea
- area
- aristotle
- armed
- armor
- artistic
- asvab
- asymptotes
- atah
- atoms
- audio
- australia
- autobiography
- autofill
- autosum
- average
- bacardi
- back
- backup
- bacteria
- bags
- balance
- banging
- baptism
- baruch
- basic
- basics
- basket
- basketball
- bass
- battery
- beans
- beds
- beef
- beer
- beginners
- being
- beliefs
- belly
- beneficiaries
- best
- betting
- between
- bidder
- bidding
- billing
- binomial
- binomials
- biogeography
- biology
- bisector
- blend
- blood
- bobbers
- boil
- bonds
- book
- bookkeeping
- borax
- borrow
- brake
- breaker
- bridge
- browsing
- build
- burn
- business
- buttocks
- button
- buying
- cables
- calculate
- calculating
- calculator
- calculus
- call
- called
- camera
- canon
- capitalized
- card
- cards
- care
- careers
- cash
- caste
- categorical
- catholic
- caulk
- caused
- causes
- centripetal
- centroid
- chalk
- change
- chart
- charts
- cheat
- check
- chemical
- chemistry
- chess
- chickens
- chord
- chords
- chromebook
- church
- circle
- circles
- circuit
- circular
- circulatory
- circumference
- class
- classical
- clean
- cleanup
- clicking
- clone
- clothes
- cloud
- club
- clubs
- coastal
- coding
- coefficients
- cogs
- cohesion
- cold
- college
- columns
- command
- commandments
- commas
- comments
- commodities
- common
- commons
- communication
- company
- compatible
- competitive
- complementary
- compost
- compute
- computer
- concrete
- conditional
- conditioning
- conduction
- conf
- confidence
- confirmation
- congruence
- congruent
- conjugating
- connect
- connecting
- connection
- cons
- constant
- constitution
- contacts
- continuous
- continuum
- contribution
- control
- controls
- convert
- cooker
- cookies
- cooking
- copper
- corinthian
- cornstarch
- cost
- costing
- cover
- craft
- crafting
- create
- critical
- crochet
- crop
- crossfit
- cubic
- current
- curtain
- curve
- cycle
- d3100
- d7000
- d7100
- data
- days
- decimal
- decimals
- decrease
- default
- define
- definition
- defrag
- defragment
- defragmenter
- degree
- delete
- deleting
- demand
- dent
- depreciation
- depression
- derivative
- derivatives
- descriptive
- design
- desktop
- deviation
- deviations
- diabetes
- diagonals
- diagram
- diamond
- diesel
- diet
- difference
- different
- differential
- dimmer
- direct
- directory
- disable
- disk
- displacement
- disposal
- distances
- distributing
- distribution
- division
- document
- does
- dogs
- domo
- down
- download
- dragon
- drainage
- drinks
- drywall
- dummies
- dural
- dutch
- earned
- earnings
- east
- eastern
- easy
- ebay
- economic
- economics
- edges
- effusion
- eggs
- elasticity
- electronics
- elements
- eleventh
- embryology
- empty
- ending
- endings
- endosymbiosis
- energy
- engine
- entries
- entry
- equation
- equations
- equilibrium
- equivalent
- estate
- ethernet
- euthanize
- evolution
- example
- examples
- excel
- exchange
- exercises
- expense
- explained
- explorer
- exponential
- exponents
- facetime
- factorial
- factory
- families
- fantasy
- fatter
- feather
- federal
- feeding
- fence
- fertilize
- fiction
- fifths
- fight
- fighting
- file
- filing
- fill
- final
- financial
- find
- fire
- first
- fish
- fishermans
- fishing
- fixed
- fixing
- flash
- flipping
- floating
- floor
- floors
- florida
- flow
- flower
- fluid
- food
- football
- footnote
- footnotes
- force
- forces
- form
- format
- formatting
- formula
- formulas
- fraction
- fractions
- frame
- free
- freeze
- freezer
- french
- frequencies
- frequency
- freshwater
- fridge
- frigidaire
- from
- fruity
- fuel
- fullback
- function
- functions
- fund
- fundamental
- furniture
- game
- garden
- gardening
- garter
- german
- gland
- glands
- glass
- glucose
- glue
- gluten
- glycemic
- gmail
- goal
- goat
- golden
- golf
- good
- goodbye
- government
- grahams
- graph
- graphing
- gravity
- grease
- great
- greek
- greetings
- grew
- groups
- guide
- guitar
- half
- handicap
- hanging
- happens
- hard
- hardwood
- heat
- heater
- hebrew
- hedge
- hierarchy
- highlight
- hinduism
- hips
- histogram
- history
- hockey
- hominy
- horse
- houses
- how to
- hydrogen
- hyperbola
- hyperlink
- hypotenuse
- icloud
- illustrator
- image
- imap
- imovie
- imperfect
- import
- improper
- impulse
- impulses
- income
- increment
- indent
- index
- indian
- indirect
- individuals
- inferential
- infrastructure
- inheritance
- inprivate
- insert
- inside
- install
- installing
- interest
- interior
- internet
- interval
- investing
- investments
- ionic
- ipad
- iphone
- ipod
- ireland
- israel
- italian
- italicize
- itunes
- jasmine
- java
- jesus
- join
- joined
- journal
- jumper
- jumping
- keeper
- kindle
- kinetic
- knit
- knitted
- knitting
- knocking
- laboratory
- lacrosse
- laptop
- latent
- latitude
- lawn
- laws
- layout
- league
- leaking
- leaks
- leasing
- left
- length
- lenses
- letter
- letterhead
- levels
- lice
- life
- light
- limits
- linear
- list
- liters
- live
- load
- local
- located
- loin
- long
- loose
- loss
- lying
- lymphatic
- macbook
- machine
- macro
- macros
- made
- madness
- main
- maintaining
- make
- management
- mandarin
- manually
- manufacturing
- many
- march
- margin
- marks
- masking
- maslows
- mass
- math
- matlab
- mean
- meaning
- measure
- measuring
- mechanism
- medical
- menu
- merchandise
- message
- metal
- metallic
- metalloid
- metalloids
- metals
- metaphysics
- microsoft
- middle
- minecraft
- minor
- mixed
- mixture
- mode
- model
- modes
- molality
- molarity
- mole
- moles
- monosaccharides
- moral
- mortal
- mosaic
- most
- mountain
- movements
- movies
- much
- muscle
- mushroom
- music
- nails
- name
- names
- natural
- naturally
- need
- needles
- needs
- nerve
- nerves
- network
- neutrality
- nexus
- nikon
- noise
- nonmetal
- nonmetals
- nonpolar
- nook
- normal
- noses
- notation
- notebook
- notes
- nspire
- numbering
- numbers
- numerical
- object
- obligation
- octagon
- odds
- often
- onedrive
- onto
- option
- options
- order
- organs
- origins
- outlook
- output
- oven
- overheats
- ownership
- page
- paint
- paints
- palestine
- pane
- paperwhite
- parabola
- parallelogram
- parathyroid
- parental
- parity
- part
- participle
- partition
- parts
- party
- past
- paste
- payable
- payout
- pegs
- percent
- percentage
- period
- periodic
- periods
- permutations
- pharynx
- phone
- photos
- photoshop
- photosynthesis
- phrases
- physics
- piano
- pipe
- pipes
- planning
- plants
- play
- playing
- playlist
- playstation
- plot
- plots
- plugs
- plunger
- poem
- poetry
- point
- points
- poisonous
- polar
- politics
- polygon
- polygons
- population
- pork
- portfolio
- position
- postulate
- potential
- powered
- powerpoint
- premium
- present
- previous
- price
- problem
- problems
- process
- processor
- profit
- project
- pronounce
- pronouns
- properly
- properties
- property
- pros
- protects
- protein
- proton
- pumped
- punctuate
- puppet
- puppies
- puppy
- pure
- quadrilateral
- quantity
- quantum
- quarterback
- quarters
- queen
- questions
- quick
- quickbooks
- quote
- racing
- radians
- radio
- raising
- range
- rank
- rationalize
- reaction
- reading
- real
- reasoning
- rebel
- recipe
- reconfigure
- record
- recorder
- rectangle
- redstone
- reference
- reflecting
- reform
- refracting
- refrigerator
- regular
- regulations
- reinstall
- relationships
- remove
- remover
- renovating
- repair
- repeater
- replacement
- replacing
- replication
- reset
- residual
- resolution
- restart
- restore
- retained
- retriever
- reverse
- rhombus
- right
- roaming
- roberts
- roku
- roman
- rooms
- rotational
- roulette
- round
- rounds
- router
- rows
- rules
- rummy
- running
- saddle
- saltwater
- sauvignon
- scalene
- schedule
- science
- scientific
- scope
- score
- scored
- scratch
- scratches
- screen
- screensaver
- scripts
- sealing
- secant
- second
- selection
- seller
- seniors
- series
- server
- service
- services
- setting
- settings
- setup
- shades
- shadow
- sharepoint
- sheet
- shooting
- shortcut
- shot
- shutter
- side
- sigma
- sign
- silicon
- silver
- simple
- singing
- siri
- skewed
- skill
- slime
- slow
- small
- snap
- soccer
- socket
- socks
- soft
- soften
- solar
- solder
- solve
- solving
- someone
- something
- song
- songs
- sonnet
- sound
- space
- spades
- spanish
- spark
- speaking
- speed
- split
- sports
- spotify
- spring
- spss
- squeaky
- standard
- start
- starting
- state
- statement
- states
- static
- statistics
- stats
- step
- stock
- stocks
- storage
- strap
- strategic
- strategy
- streetlights
- string
- styles
- subsetting
- substance
- sugar
- sugars
- summary
- sums
- supplementary
- support
- switch
- swype
- symbols
- sync
- synthesis
- system
- table
- tablet
- tactical
- take
- tangent
- tank
- taxes
- team
- telescope
- temperature
- tennis
- tense
- term
- terms
- test
- testate
- testing
- tether
- their
- theorem
- theory
- theta
- thigh
- things
- through
- tile
- time
- timeline
- timer
- timing
- tips
- toilet
- tone
- tool
- tools
- torch
- toshiba
- tour
- trading
- training
- transfer
- transitive
- trash
- triangle
- triangles
- tricks
- trig
- trigonometry
- trim
- troubleshoot
- troubleshooting
- truck
- truss
- tune
- tuners
- turn
- tutorial
- type
- types
- typing
- unallocated
- unclog
- uncollectible
- under
- understanding
- undo
- unfriending
- unhide
- uninstall
- united
- unix
- upgrade
- urinary
- using
- value
- values
- vaporization
- variable
- variables
- vegan
- verb
- verbs
- video
- view
- violin
- vista
- vocals
- voicemail
- volume
- voting
- waist
- wake
- warp
- washing
- water
- watermarks
- wedding
- week
- weight
- were
- wether
- what
- whats
- when
- where
- which
- whisker
- width
- wifi
- wilsons
- win7
- window
- windows
- wine
- winning
- winxp
- wire
- wireless
- with
- wont
- wooden
- woodrow
- word
- work
- working
- workplace
- world
- wrestling
- write
- writing
- xbox
- yarn
- your
- zeros
- zoom
-
Adjust the Truss Rod 1. Decrease Relief Tightening the truss rod by turning it clockwise influences the neck to curve upward toward the stri...
-
Divide this by 100 to get 68 which means 68 is 20 of 340. As a fraction 10 200 005. Percentages Fast Math Lesson Youtube 10 200 x 100 5. ...
-
Double-entry bookkeeping is the process that most businesses use to produce their accounts. Double entry bookkeeping is where the value from...
